Which of the following graphs is correct for a zero order of reaction?
A

B

C

D

Text Solution
The correct Answer is:A, D
|
Topper's Solved these Questions
- CHEMICAL KINETICSBook:DINESH PUBLICATIONChapter:CHEMICAL KINETICSExercise:Multiple type questions
Explore 4 Videos
- CHEMICAL KINETICSBook:DINESH PUBLICATIONChapter:CHEMICAL KINETICSExercise:Assertion Reason type questions
Explore 5 Videos
- CHEMICAL KINETICSBook:DINESH PUBLICATIONChapter:CHEMICAL KINETICSExercise:Higher order thinking skills (hots) question
Explore 18 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:DINESH PUBLICATIONChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Unit Test - 1
Explore 20 Videos
- CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS Book:DINESH PUBLICATIONChapter:CHEMICAL THERMODYNAMICS AND CHEMICAL ENERGETICS Exercise:Exercise
Explore 507 Videos
Similar Questions
Knowledge Check
Which of the following graphs is correct for a zero order reaction ?
A B
B C
C D
D
Which of the following graph is correct for zero order reaction?
A B
B C
C D
D
Which of the following graph is correct for a first order reaction ?
A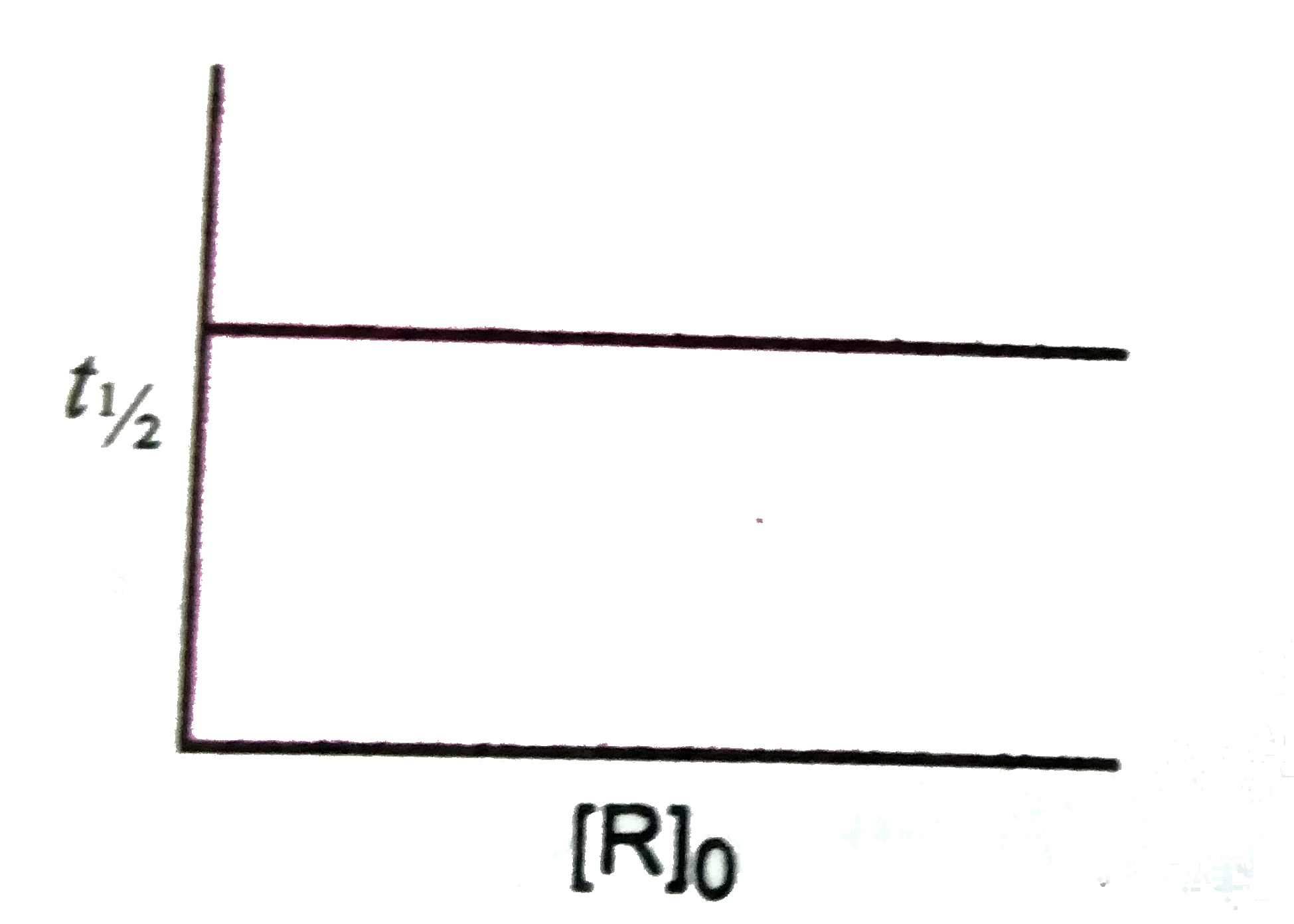 B
B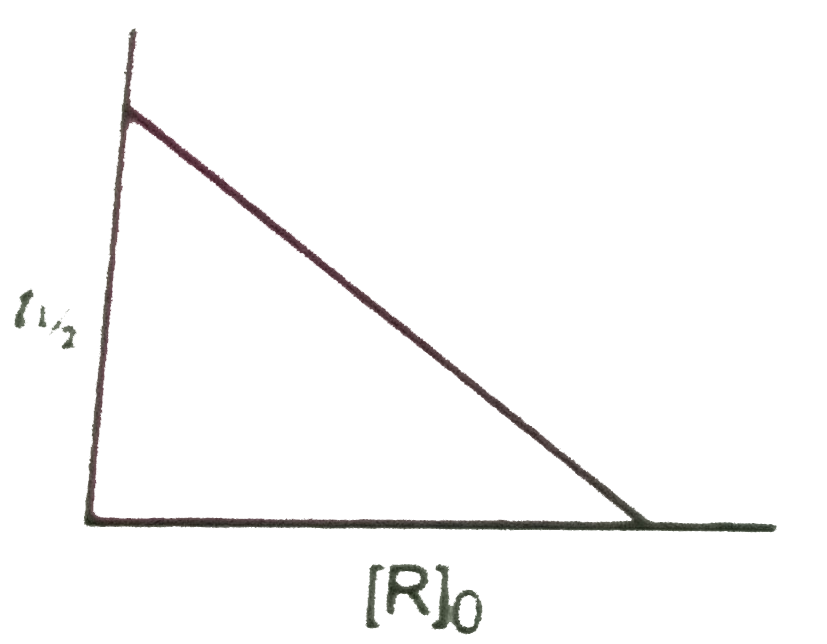 C
C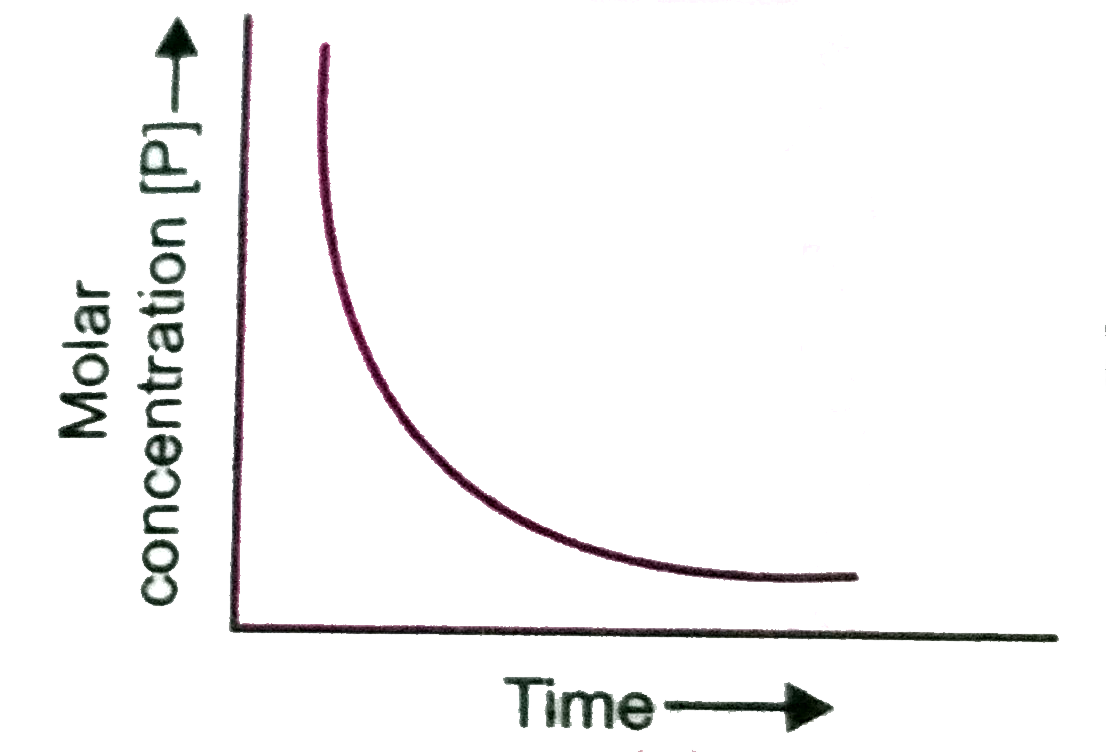 D
D
Which of the following graph is correct for a first order reaction ?
View SolutionWhich of the following graphs is correct for a first order reaction?
View SolutionWhich of the following graphs is correct for a first order reaction ? (R→P)
View SolutionWhich of the following graph is correct for a first order reaction ?
View SolutionWhich of the following graphs is correct for second order reaction ?
View Solution

