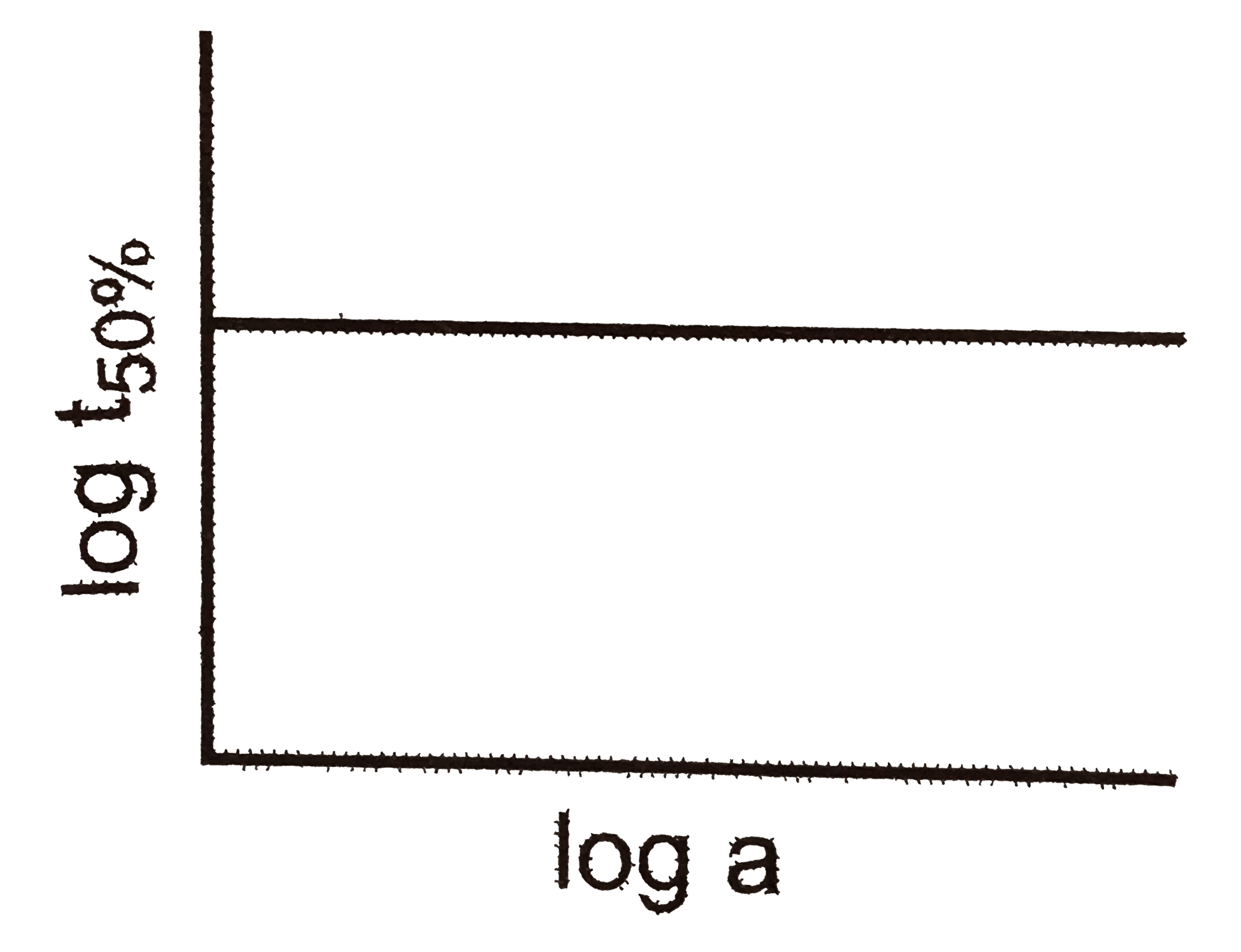
A graph plotted between logt50% vs log concentration in a straight line. What conclusion can you draw from this graph?

A
n= 1, t1/2=tx×a
B
n=2,t1/2=1a
C
n=1,t1/2=0.693k
D
none of these
Text Solution
The correct Answer is:C
c) t1/2∝a1−n=k(a)1−n
log t1/2=logk+(1−n)loga
The graph is expected to be a straight line.
Therefore, slope 1-n =0 or n=1
|
Similar Questions
Knowledge Check
A graph plotted between log t50% vs log concentration is a straight line. What conclusion can you draw from this graph?
 An=1:t1/2=1x×aBn=2:t1/2=1aCn=1:t1/2=0.693kDNone of these
An=1:t1/2=1x×aBn=2:t1/2=1aCn=1:t1/2=0.693kDNone of theseA graph plotted between log t50% vs log concentration is a straight line. What conclusion can you draw from this graph?
 Aa=1:t1/2∝aBa=2,t1/2∝1/aCa=1:t1/2=(0.693/k)DNone of these
Aa=1:t1/2∝aBa=2,t1/2∝1/aCa=1:t1/2=(0.693/k)DNone of theseA graph plotted between log t50% vs log concentration is a straight line. What conclusion can you draw from this graph?
 Aa=1:t1/2∝aBa=2,t1/2∝1/aCa=1:t1/2=(0.693/k)DNone of these
Aa=1:t1/2∝aBa=2,t1/2∝1/aCa=1:t1/2=(0.693/k)DNone of these
A graph plotted between logt12 vs log concentration is a straight line. What is your conclusion?
[Given : n = order of reaction]
View SolutionA graph plotted between log t50% vs log concentration is a straight line. What conclusion can you draw from this graph?

View SolutionA graph plotted between log k vs 1T is represented by
View SolutionA graph plotted between logk versus 1/T for calculating activation energy is shown by
View SolutionA graph plotted between log N vs, time gives a slope and intercept equal to:
View Solution


