The following data were obtained during the first order decomposition of SO2Cl2 at a constant volume:
SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate constant.
SO2Cl2(g)→SO2(g)+Cl2(g)
The decomposition reaction is in the gaseous phase. The expression for the rate constant is:
k=2.303tlog(pi)2π−pt
t=100s,π=0.4atm,pt=0.7atm
k=2.303100slog[0.4atm(0.8−0.7)atm]
k=2.303100slog4=2.303100s×0.6020=1.39×10−2s−1
Similar Questions
The following data were obtained during the first order thermal
decomposition of SO2Cl2 at a constant volume SO2Cl2(g)→SO2(g)+Cl2(g)
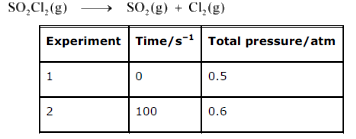
Calculate the rate of the reaction when total pressure is 0.65 atm
View SolutionThe following data were obtained during first order thermal decomposition of SO2(Cl2) at a constant volume.
SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate of the reaction when the total pressure is 0.65 atm.
View Solution
Knowledge Check
The following data were obtained during the first order thermal
decomposition of SO2Cl2 at a constant volume SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate of the reaction when total pressure is 0.65 atmA0.35atmS−1B2.235×10−3atmS−1C7.8×10−4atms−1D1.55×10−4atmS−1The following data were obtained during the first order thermal decomposition of SO2CI2 at a constant volume.
SO2CI2(g)→SO2(g)+CI2(g)
ExperimentTime/s−1Total pressure/atm100.521000.6
What is the rate of reaction when total pressure is 0.65 atm?A0.35atms−1B2.235×10−3atms−1C7.8×10−4atms−1D1.55×10−4atms−1
The following data were obtained during the first order thermal decomposition of N2O5(g) at constant volume.
2N2O5(g)→2N2O2(g)+O2(g)

Calculate the rate constant for the gaseous reaction.
View SolutionThe following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume.
SO2Cl2(g)→SO2(g)+Cl2(g)
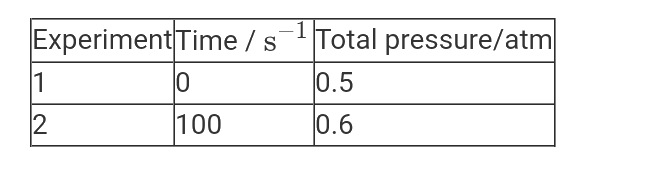
What is the rate of reaction when total pressure of 0.65 atm?
View SolutionThe following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume :
SO2Cl2(g)→SO2(g)+Cl2(g)
Experiment Times/s−1 Total pressure/atm1 0 0.42 100 0.7
Calculate the rate constant. Given : log 4 = 0.6021, log 2 = 0.3010)
View SolutionThe following data were obtained during the first order thermal
decomposition of SO2Cl2 at a constant volume SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate of the reaction when total pressure is 0.65 atm
View SolutionThe following data were obtained during the first order thermal decompostion of SO2CI2 at a constant volume :
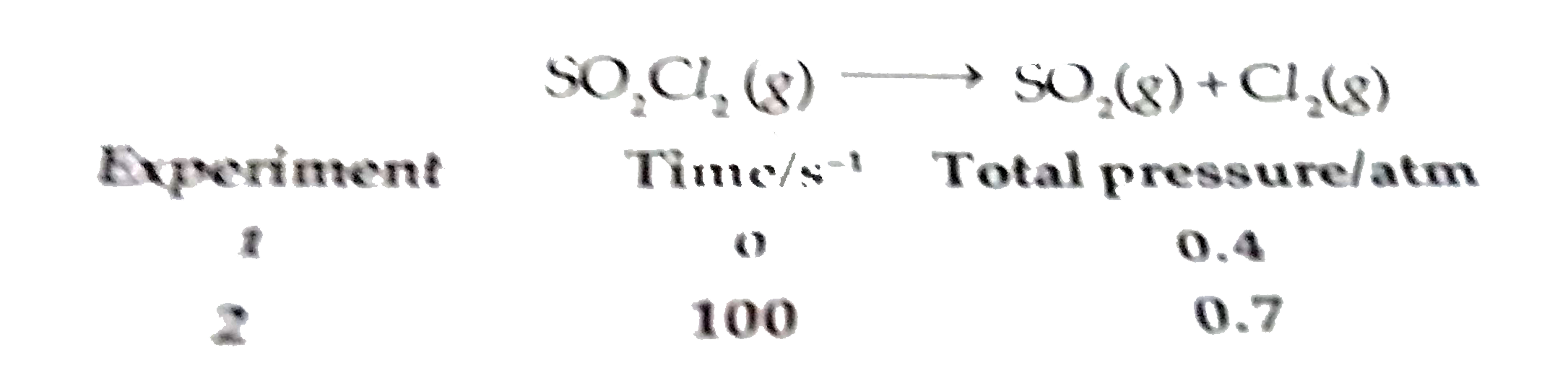
Calculate rate constant
View SolutionThe following data were given for thermal decomposition of SO2Cl2 at a constant volume :
SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate of the reaction when total pressure is 0.65 atm.
View Solution

