Which of the following sets of quantum numbers is correct for an electron in 3d orbital.Solution in Telugu
A
n=3,1=2,m=−3,s=+12
B
n=3,1=3,m=+3,s=−12
C
n=3,1=2,m=−3,s=−12
D
n=3,1=2,m=−3,s=−12
Video Solution
Text Solution
The correct Answer is:c
For 3d orbital containing single electron,the orbital diagram is
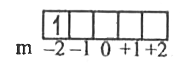
n=3,2,m=−2,s−+1/2
|
Similar Questions
Which of th e following represents the correct set of four quantum number of 3d orbital electron ?
View Solutionएक इलेक्ट्रॉन 3p कक्षक में है तो इस इलेक्ट्रॉन के लिए क्वाण्टम संख्या का कौन सा समुच्चय सही है।
View SolutionList the quantum numbers (n and l) of electrons for 3d-orbital.
View SolutionWrite the quantum number of an electron present in 3d-orbital.
View SolutionThe Correct set of four quantum numbers for an 3d electron—
View SolutionWhich one of the following sets of quantum number is possible for electron in a 4f- orbital?
View SolutionWhich one of the following sets of quantum number is possible for electron in a 4f- orbital?
View SolutionList the quantum numbers m_l and l of electron in 3d orbital.
View Solution

