Identify the correct statements from the following.
(1) The dipole moment of CO2andBF3 is zero
(2) The dipole moment of NF3 is higher than the diople moment of NH3
(3) The dipole moment of HI is lower than the dipole moment of HClSolution in Telugu
A
1, 3
B
1, 2
C
2, 3
D
1, 2,3
Video Solution
Text Solution
The correct Answer is:A
1. The BF3 molecule has a symmetrical trigonal planar geometry, the moment of any two B-F dipoles is equal in magnitude but opposite in direction to the moment of the BF3 molecule is zero. Same as CO2.
2. In NH3, N is more electronegative than H so, N pulls the electrons from H towards itself so, the direction of moment due to the N-H bonds is in the same direction as that of the long pair of electrons on nitrogen.
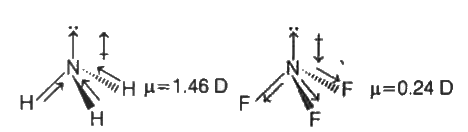
But in case of NF3,H∗ [lone pair] moment is in the opposite direction to the N-F bond moments.
Thus, has smaller net dipole moment.
3 ∴ Size of I-atom is more than of Cl-atom and electronegativit of I-atom is less than of Cl-atom. Hence, HI has lower dipole -moment than HCl.
|
Similar Questions
The dipoles moment of NF3 is less than NH3 because
View SolutionOut of NH3 and NF3 which has a higher Dipole moment?
View SolutionWhy is dipole moment of CO_(2)' BF_(3), CCl_(4) is zero?
View Solutiona) Discuss the significance/applications of dipole moment.
b) Represent diagrammatically the bond moments and the resultant dipole moment in CO2,NF3 and CHCl3
View SolutionThe dipoles moment of NF3 is less than NH3 because
View SolutionNF3 का द्विध्रुव आघूर्ण NH3 से कम है क्योंकि
View SolutionNH3 के द्विध्रुव आघूर्ण का कुछ मान होता है जबकि BF3 का द्विध्रुव आघूर्ण शून्य होता है क्योंकि
View SolutionDipole moment of NF3 is smaller than :
View Solution
