Statement (I) Co2+ has higher magnetic moment than Cr3+
Statement (II) Ionisation enthalpies of Ce, Pr and Nd are higher than Th, Pa, U
Which of the following is correct ?Solution in Telugu
A
Both (I) and (II) are not correct
B
Both (I) and (II) are correct
C
(I) is correct but (II) is not correct
D
(I) is not correct but (II) is correct
Video Solution
Text Solution
The correct Answer is:D
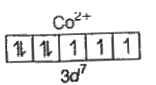
μ=√n(n+2)n=3sqr15=3.95B.M
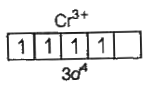
μ=√n(n+2)=√24=4.95B.M
Statement (I) is not correct as magnetic moment of Cr3+ is more than of CO2+. But, statement (II) is correct, as I.P. of Ce. Pt and Nd are higher than th, Pa and U. Thus, the correct option is (d).
|
Similar Questions
The first ionisation enthalpy of Mg is higher than the first ionisation enthalpy of:
View SolutionWhy ionisation enthalpy of TI is higher than In?
View SolutionIonisation enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
View SolutionIonisation enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
View SolutionWhy is the ionisation enthalpy of hydrogen higher than that of sodium ?
View SolutionCe, Pr और Nd की आयनन एन्थैल्पियाँ, Th, Pa और U अपेक्षा उच्च होती है। क्यों?
View SolutionExplain the following :
Gallium has higher ionisation enthalpy than aluminium .
View SolutionWhy the ionization energy of Ce, Pr, Nd is higher than Th, Pa and 0. it occurs ?
View Solution

