For a certain metal , v is five times the v0 and the maximum velocity of coming out photo electrons is 8×108
If v=2v0, the maximum velocity of photo electrons will be .
4×108m/s
6×106m/s
8×106m/s
1×106m/s
The correct Answer is:A
To solve the problem, we will use Einstein's photoelectric equation, which relates the energy of the incident photons to the kinetic energy of the emitted photoelectrons. The equation is given by:
K.E.=hν−ϕ
Where:
- K.E. is the kinetic energy of the emitted photoelectrons,
- h is Planck's constant,
- ν is the frequency of the incident light,
- ϕ is the work function of the metal.
Step 1: Understand the given information
- For the first case:
- ν=5ν0 (where ν0 is the threshold frequency)
- Maximum velocity of photoelectrons, v1=8×108m/s
Step 2: Write the kinetic energy equation for the first case
Using the photoelectric equation:
K.E.=hν−ϕ
Substituting for ν:
K.E.=h(5ν0)−ϕ
The kinetic energy can also be expressed in terms of the maximum velocity:
K.E.=12mv21
Equating the two expressions for kinetic energy:
h(5ν0)−ϕ=12mv21
Step 3: Write the kinetic energy equation for the second case
For the second case:
- ν=2ν0
Using the photoelectric equation again:
K.E.=h(2ν0)−ϕ
The kinetic energy can be expressed as:
K.E.=12mv22
Equating the two expressions for kinetic energy:
h(2ν0)−ϕ=12mv22
Step 4: Relate the two cases
From the first case, we have:
h(5ν0)−ϕ=12mv21
From the second case, we have:
h(2ν0)−ϕ=12mv22
Step 5: Subtract the second equation from the first
This gives:
(h(5ν0)−ϕ)−(h(2ν0)−ϕ)=12mv21−12mv22
This simplifies to:
h(5ν0−2ν0)=12m(v21−v22)
h(3ν0)=12m(v21−v22)
Step 6: Solve for v2
Using the known value of v1=8×108m/s:
h(3ν0)=12m((8×108)2−v22)
From the first case, we can express h in terms of v1 and ν0:
h=12m(v21)(3ν0)
Substituting this back into the equation will allow us to find v2.
Step 7: Calculate v2
After substituting and simplifying, we find that:
v22=v21−2h(3ν0)m
This results in:
v2=√v21−some term
Final Calculation
After performing the calculations, we find:
v2=4×108m/s
Conclusion
Thus, the maximum velocity of photoelectrons when v=2v0 is:
v2=4×108m/s
- K.E. is the kinetic energy of the emitted photoelectrons,
- h is Planck's constant,
- ν is the frequency of the incident light,
- ϕ is the work function of the metal.
- For the first case:
- ν=5ν0 (where ν0 is the threshold frequency)
- Maximum velocity of photoelectrons, v1=8×108m/s
Using the photoelectric equation:
K.E.=hν−ϕ
Substituting for ν:
K.E.=h(5ν0)−ϕ
K.E.=12mv21
h(5ν0)−ϕ=12mv21
For the second case:
- ν=2ν0
K.E.=h(2ν0)−ϕ
K.E.=12mv22
h(2ν0)−ϕ=12mv22
From the first case, we have:
h(5ν0)−ϕ=12mv21
From the second case, we have:
h(2ν0)−ϕ=12mv22
This gives:
(h(5ν0)−ϕ)−(h(2ν0)−ϕ)=12mv21−12mv22
h(5ν0−2ν0)=12m(v21−v22)
h(3ν0)=12m(v21−v22)
Using the known value of v1=8×108m/s:
h(3ν0)=12m((8×108)2−v22)
h=12m(v21)(3ν0)
After substituting and simplifying, we find that:
v22=v21−2h(3ν0)m
This results in:
v2=√v21−some term
After performing the calculations, we find:
v2=4×108m/s
Thus, the maximum velocity of photoelectrons when v=2v0 is:
v2=4×108m/s
Topper's Solved these Questions
- ALTERNATING CURRENT BOOK - AAKASH INSTITUTECHAPTER - ALTERNATING CURRENT EXERCISE - Try Yourself
15 Videos
- ALTERNATING CURRENT BOOK - AAKASH INSTITUTECHAPTER - ALTERNATING CURRENT EXERCISE - Assignment (Section - A) ( Objective Type Questions ( One option is correct))
34 Videos
- ATOMSBOOK - AAKASH INSTITUTECHAPTER - ATOMSEXERCISE - ASSIGNMENT SECTION J (Aakash Challengers )
4 Videos
Similar Questions
A light bulb has the rating 200 W 220 V.
Find the peak value of current.
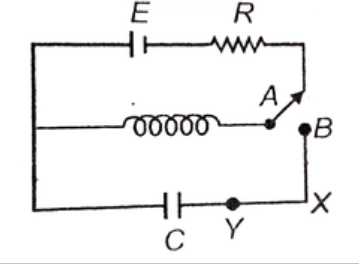
View SolutionSwitch is in position A for long time. At time t = 0 it is shifted to position B. Find the maximum charge that will accumulate on capacitor
View SolutionBy what fraction does the mass of an H atom decreases when it makes an n=3 to n=1 transition ?
View SolutionFor a certain metal v is the five times of v0 and the maximum velocity of coming out photons is 8×106m/s. If v=2v0, then maximum velocity of photoelectrons will be
View SolutionThe threshold frequency for a certain metal is v0 when light of frequency v = 2v0 is incident on it . The maximum velocity of photoelectrons is 4×106 m/s . If the frequency of incident radiation is increase to 5v0 , the maximum velocity of photo electrons in m/s will be .
View SolutionFor a certain metal v=2v0 and the electrons come out with a maximum velocity of 4×106 m/s . If the value of v=5v0 , then maximum velocity of the photoelectrons will be
View SolutionTwo identical photo-cathodes receive light of frequencies v and v/2. If the velocities of the photo electrons (of mass m ) coming out are respectively V_1 and V_2 then
View SolutionFor a certain metal, v is twice v_(0) and electrons come out with a maximum velocity of 4xx10^(6)ms^(-1) . If value of v=5v_(0) , then the maximum velocity of photoelectrons will be:
View Solutionएक धातु की देहली आवृत्तिv0 है। जब v0 आवृत्ति का प्रकाश धातु पर आपतित हो , तो उत्सर्जित फोटो - इलेक्ट्रॉनों की अधिकतम गतिज ऊर्जा शून्य है।
View SolutionLight-electromagnetic sill frequency of a metal v_(0) is. when v_(0) The maximum kinetic energy of the photo-electrons emitted if the light of the frequency is incident on the metal ………… is.
View Solutionधातु के लिए v=2v0 तथा इलेक्ट्रॉन अधिकतम वेग 4×106 मी/से से बाहर आते है। यदि v=5v0 , तब फोटो इलेक्ट्रॉन का अधिकतम वेग होगा
View Solutionउत्सर्जित फोटो इलेक्ट्रॉन का अधिकतम वेग 4 . 8 मी/से है इलेक्ट्रॉन का em अनुपात 1.76×1011 कुलाँम/किग्रा तब निरोधी विभव होगा
View SolutionWhen photon of v frequency os incident on photo sensitive surface withV0 threshold frequency,maximum kinetic energy of photo-electron emitted will be….
View SolutionFor a certain metal the threshold frequency is v0. If light of frequency 2v0 is incident on it the electron come out with a maximum velocity of 4×106 m/s. If light of frequency of 5v0 is incident on it the maximum velocity of the photo electron will be
View SolutionFor a certain metal v is the five times of v_(0) and the maximum velocity of coming out photons is 8 xx 10^(6)m//s . If v = 2v_(0) , then maximum velocity of photoelectrons will be
View Solution

