Which of these elements have almost same electronegativity?
(i) C,I (ii) P, H (iii) N, Cl (iv) Br,S
The correct Answer is:Phosphorous and hydrogen have almost similar electronegativity values of 2.1. Similarly carbon and iodine have a value of 2.5; Nitrogen and Chlorine have a value of 3.0 and Bromine, Sulphur have a value of 2.8.
Step by step video, text & image solution for Which of these elements have almost same electronegativity? (i) C,I (ii) P, H (iii) N, Cl (iv) Br,S by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
What is the order of polarity of the following bond?
(i) C-H (ii) F-H (iii) Br -H
(iv) Na-I (v) K-F (vi) Li-Cl
View SolutionAmong the following , the pair of elements having same electronegativity values are
A) (H,P) B) (Be , Al)
C) (N,Cl) D) (C,P)
View SolutionMatch Column A with Column B
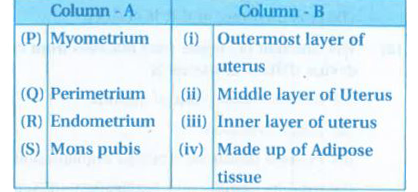
(A) (P-i), (Q-ii), (R-iv), (S-iii)
(B) (P-ii), (Q-i), (R-iii), (S-iv)
(C) (P-ii), (Q-iii), (R-iv), (S-i)
(D) (P-iv), (Q-iii), (R-ii), (S-i)
View SolutionMatch the following (A) (i-P), (ii-S), (iii-R), (iv-Q) (B) (i-Q), (ii-S), (iii-P), (iv-R) (C) (i-Q), (ii-P), (iii-R), (iv-S) (D) (i-P), (ii-R), (iii-Q), (iv-S)
View Solutionकिसका जल अपघटन नहीं होता है
(i) Cl− (ii) NO−3 (iii) Br− (iv)ClO−4
View SolutionArrange increasing order of atomic radius of given elements.
(i) O, N, F, B, Be, Li, C br> (ii) P, S, Mg, Na, Al, Si, Cl
(iii) K, Na, Li, Cs, Rb
(iv) Cl, F, I, At, Br
View SolutionThe decreasing basic order of the following is: i. F^(o-) , ii. Cl^(o-) , iii. Br^(o-) , Iv. I^(o-)
View SolutionWhat is the order of polarity of the following bond? (i) C-H (ii) F-H (iii) Br-H (iv) Na-I (v) K-F (vi) Li-Cl
View Solution
