The hydroboration oxidation of 2-methyl propene yields-
A
1∘ alcohol
B
2∘ alcohol
C
3∘ alcohol
D
None
Video Solution
Text Solution
The correct Answer is:A
CH3−C∣CH3H=CH2(i).BH3−THF/HOH−−−−−−−−−−−−−−→(ii).H2O2/NaOHCH3−CH−OH∣CH21∘−Alcohol
This is a method of additio of HOH molecule by Anti-Markovnikov's Rule.
|
Similar Questions
The major product formed during the hydroboration-oxidation of 1-methylcyclopentene is-
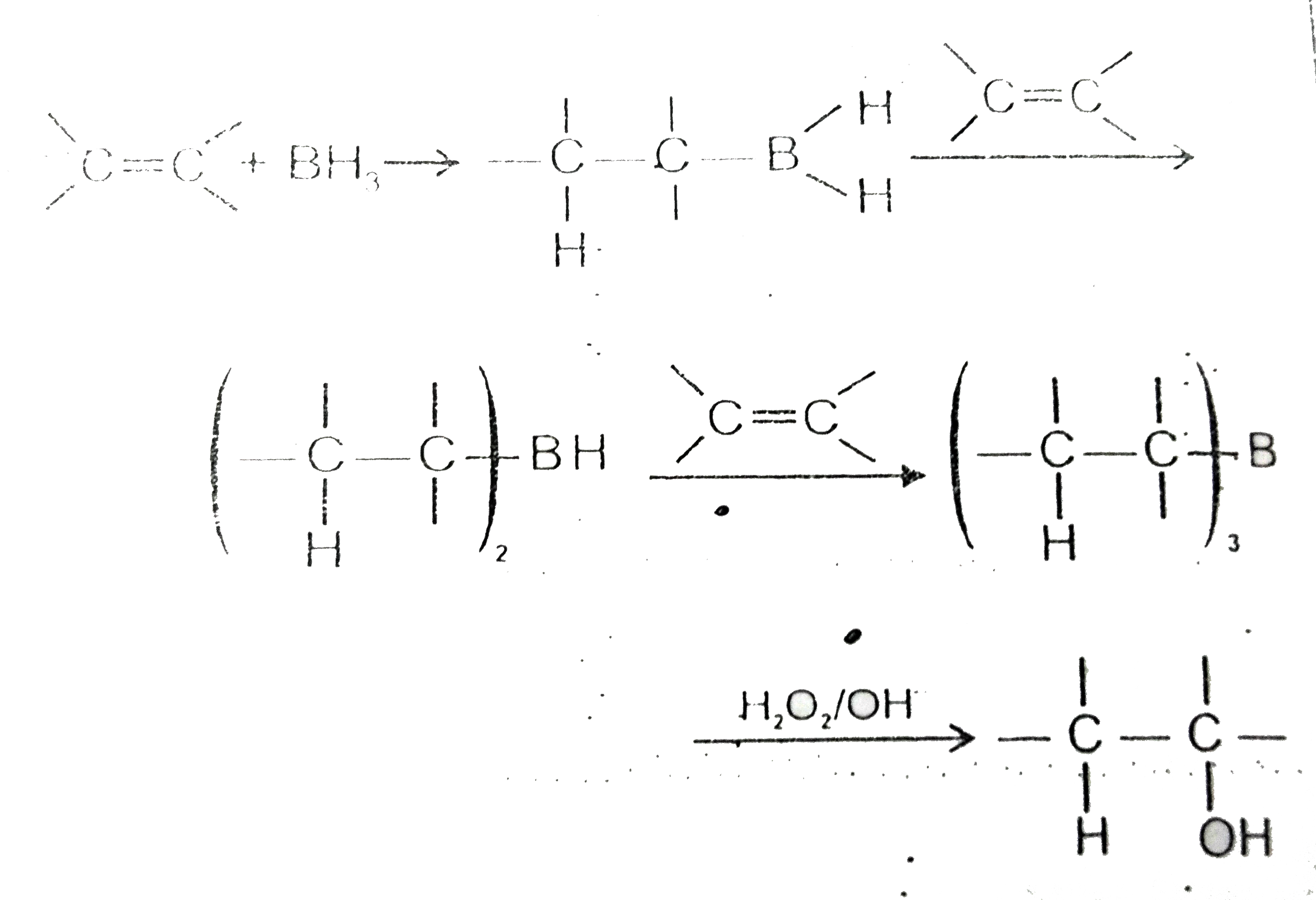
View SolutionAddition of water molecule across double bond to yield Antimarkownikov's product. Can be accomplished by Hydroboration followed by oxidation. Reaction follows as:
Product of hydroboration oxidation of 1-methyl cyclopentene is
View Solution
Knowledge Check
Hydroboration oxidation of 4-methyl octene would give
A4-methyl octanolB2-methyl decaneC4-methyl heptanolD4-methyl-2-octanoneHydroboration oxidation of 3-methyl but-1-ene gives
A3-methylbutan-2-olB2-methylbutan-2-olC3-methylbutan-1-olD2-methylbutan-1-olThe major product formed during hydroboration oxidation of 1-methylcyclopentene is
A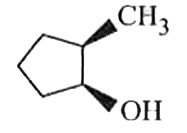 B
B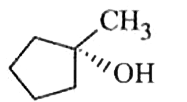 C
C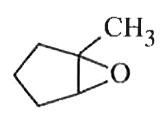 D
D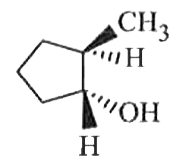
Hydroboration oxidation of propene produces
View SolutionHydroboration oxidation of 4-methyl octene would give
View SolutionHydroboration oxidation of alkene give
View Solution
Which of the following is true regarding the hydroboration oxidation of alkene ?
View SolutionIsobutylene when subjected to hydroboration oxidation reaction yields
View Solution
