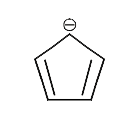
How many π electrons are there in a planar ring of phenol
A
4
B
6
C
8
D
10
Video Solution
Text Solution
The correct Answer is:C
Electron in π bonds are π electrons & lone pair electrons which delocalises in the process of resonance are also counted as π electrons
|
Similar Questions
Knowledge Check
How many electrons are there in Na+
A1B10C11D12How many electrons are there in Li2?
A4B6C5D3How many electrons are there in one coulomb of electricity?
A6.02×1021B6.02×1016C6.24×1018D6.24×1015
How many valence electrons are there in each of these elements?
View SolutionHow many valence electrons are there in each of these elements?
View Solution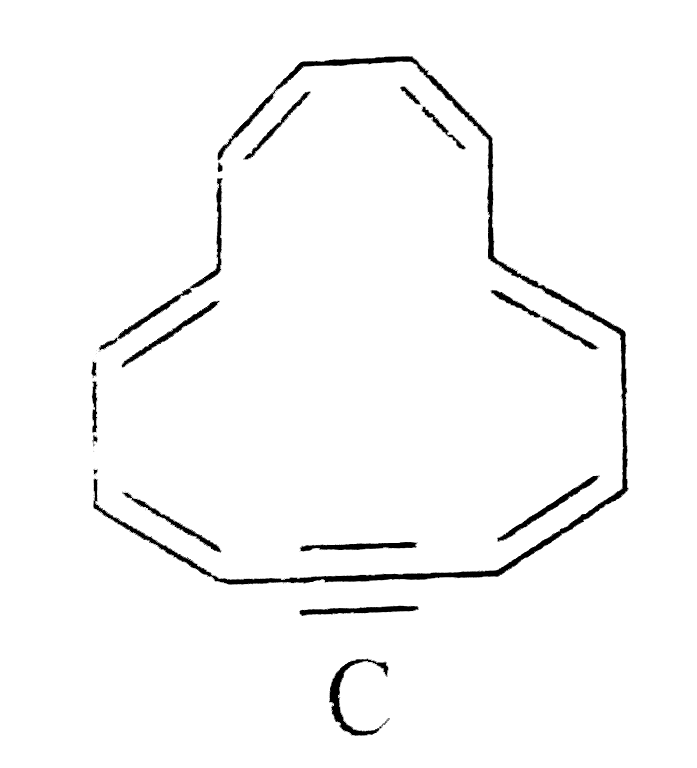
(a) How many π electrons does C contion?
(b) How many π electrons are delocalized in the ring?
Explain why C is aromatic
View SolutionHow many π electrons are there in the following species ?
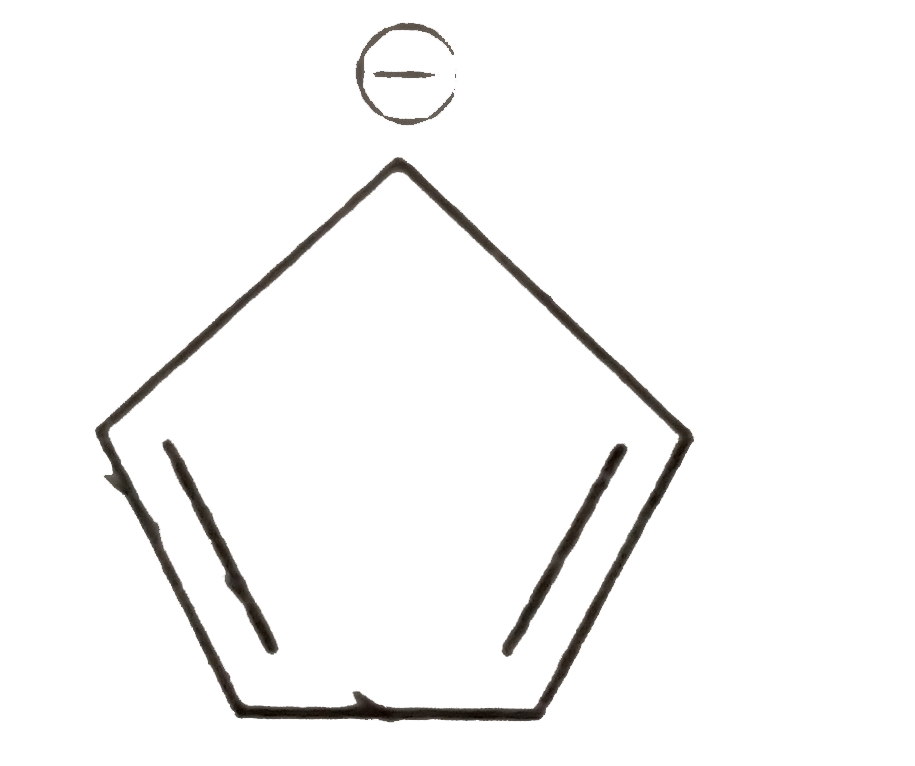
View Solutionhow many π electrons are there in the following species ?

View Solution