The enthalpy of gas phase trimerization of one mole of gaseous formaldehyde in (kJ/mole)

The correct Answer is:A
ΔH=∈C=O−2∈C−O ⇒700−720=−20kJ/mole
Similar Questions
The enthalpy change of formation of CO2(g) is -393 kJ mol−1 and that of H2O(l) is -286 kJ mol−1. The enthalpy of combustion of one mole of ethanol (C2H5OH) is -1360. the enthalpy change for the formation of one mole of ethanol form its constituent elements is
View SolutionOne mole of a gas is defined as:
View Solution
Knowledge Check
The enthalpy of gas phase trimerzation of one mole of gaseous formaldehyde in (KJ/mole)
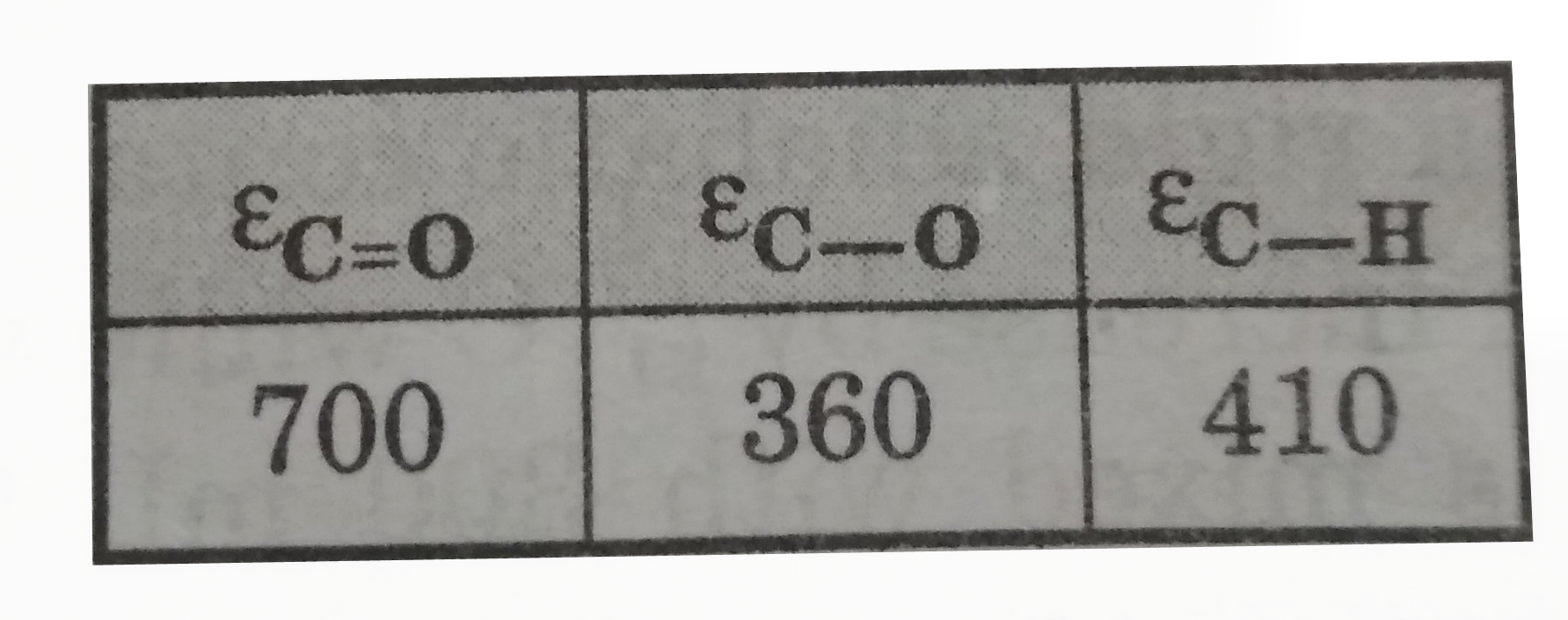
Bond energies (kj/mole)
Given:
εc=oεc−oεc−H700360410A−20B−60C−10D−50The internal energy of one mole of a gas is
A32RTB12kTC12RTD32kTThe enthalpies of vombustion of formaldehyde and paraformaldehyde (a ploymer of formaldehyde) arae -134 and -732 kcal/mol respectively .The enthalpy of polymerisation per mole of paraformaldehtde is -72 kcal the molecular formula of paraformaldehyde is:
ACH2OBC6H12O6Cc12H22O11DC3H6O3
The kinetic energy of one mole of an ideal gas
View SolutionThe kinetic energy of one mole of an ideal gas
View SolutionThe enthalpy of tetramerization of X in gas phase (4X(g)→X4(g)) is -100 kJ/mol at 300 K. The enthalpy of vaporisation for liquid X and X4 are respectively 30 kJ/mol and 72 kJ/mol respectively. ΔS for tetramerization of X in liquid phase is -125 J/K mole at 300 K. What is the ΔG at 300 K for tetramerization of x in liquid phase.
View SolutionThe value of ΔHO−H is 109Kcal/mol . The enthalpy of formation of 1 mole of water in gaseous state will be
View SolutionConsider the following reaction between zinc and oxygen
2Zn(s)+O2(g)→2Zn(s):ΔH=−693.8kjmol
I The enthalpy of two moles of is less than total enthalpy of two moles of Zn and one mole of oxygen by 693.8
The enthalpy of two moles of ZnO is more than the total enthalpy of two moles of Zn and one mole of oxygen by 693.8 kj
693Kj /mol energy is evolved in the reaction
IV 693.8 kj/mol energy is absorbed in the reaction
Choose the correct option out of the option given above.
View Solution

