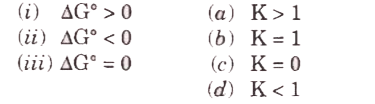The correct relationship between free energy change in a reaction and the corresponding equilibrium
constant K is
A
−ΔG∘=RTlnK
B
ΔG=RTlnK
C
−ΔG=RTlnK
D
ΔG∘=RTlnK
Text Solution
The correct Answer is:A
ΔG∘=−RTInK
|
Similar Questions
Knowledge Check
The correct relationship between free energy change in a reaction and the corresponding equilibrium constant Kc is
AΔG=RTlnKcBΔG=−RTlnKcCΔG∘=RTlnKcDΔG∘=−RTlnKcThe correct relationship between free energy change in a reaction and the corresponding equilibrium constant Kc is:
AΔG∘=RT In KCB−ΔG∘=RT In KCCΔG=RT In KCD−ΔG=RT In KCThe correct relationship between free energy change in a reaction and the corresponding equilibrium constant Kc is:
AΔG=RTlnKcB−ΔG=RTlnKcCΔG∘=RTlnKcD−ΔG∘=RTlnKc
Explain the relationship between Gibb's standard energy change of the reaction and equilibrium constant.
View SolutionThe correct relationship between free energy change in a reaction and the corresponding equilibrium constant Kc is:
View SolutionThe correct relationship between free energy change in a reaction and the corresponding equilibrium constant Kc is:
View SolutionThe correct relationship between free energy change in a reaction and the coresponding equilibrium constant K is :
View SolutionThe correct relationship between Gibb's free energy change and the EMF of a cell is
View Solution