Mass of H2O present in air in 10 lit. closed vessel with 80% relative humidity at 1 atm and 400 K ? [Vapour pressure of water at 300 K = 0.04 atm]
0.18 gm
0.36 gm
0.09 gm
0.9 gm
The correct Answer is:A
RH=0.8=PH2O(g)V.P.ofH2O=PH2O(g)0.04
PH2O(g)=0.8×0.04
PV = nRT for water wapour
0.8×0.04×10=nH2O×0.0810×400
nH2O=0.01
WH2O=0.01×18=0.18gm
Similar Questions
A container with a volume of 20.0L holds N2(g) and H2O(l) at 300K and 1.0 atm. The liquid water is then decomposed completely into H2(g) and O2(g) by any means, at constant temperature. If the final pressure becomes 1.86 atm, what was the mass of water (in gm) present initially. Neglect the initial volume of water. [Given : Vapour pressure of water at 300 K = 0.04 atm, R = 0.08 L-atm/K-mol]
View SolutionA container with a volume of 20.0 L holds N2(g) and H2O(l) at 300 K and 1.0 atm. The liquid water is then decomposed completely into H2(g) and O2(g) by any means, at constant temperature, if the final pressure becomes 1.86 atm, what was the mass of water (jn gm) present initially. Neglect the initial volume of water: [Given : vapour pressure of water at 300 K=0.04 atm L-atm / K -mol]
View Solution
Knowledge Check
What is the density of wet air with 75% relative humidity at 1 atm and 300 K? Given : vapour pressure of H2O is 30 torr and average molar mass of air is 29 g mol_1 .
A1.614 g/LB0.96 g/LC1.06 g/LD1.164 g/LWhat is the density of wet air with 40% relative humidity at 1 atm and 284.5 K. [Given vapour pressure of H2O=47.5 torr, Average molar mass of dry air = 29 g mol−1]
A1.15 g/LB1.23 g/LC1.35 g/LD1.4 g/LOn a humid day, the mole fraction of water vapour in air at 298 K is 0.0265 bar. If total vapour pressure of air is 0.980 bar and vapour pressure of water at 298 K is 0.0315 bar, the relative humidity is
A82.5B88.6C76.4D28.5
A sample of pure CuO was reduced with H2 gas and H2O formed was collected in a 44.8 L flask containing dry N2. At 27∘C, the total pressure containing N2 and H2O was 1.0 atm. The relative humidity in the flask was 80% The vapour pressure of water at 27∘C is 25 mm. How many grams of CuO was reduced?
View SolutionFind the mass of water vapour per cubic metre of air at temperature 300K and rekative humidity 50%. The saturation vapour pressure at 300K is 3.6kPa and the gas constant R=8.3JK−1mol−1.
View SolutionOn a certain humid day, the mole fraction of water vapour in air at 25∘C is 0.0287. If total pressure of air is 0.977 bar, calculate the partial pressure of water vapour in air and relative humidity if vapour pressure of water at 25∘C is 0.0313 bar.
View SolutionOne mole of CO2 at 300 K and1 atm pressure is heated in a closed vessel so that temperature is 500 K is pressure is 5 atm. Then it is cooled so that temperature is 300 K and pressure is 1 atm. What is the change in internal energy of the gas ?
View SolutionA gas 'X' is present with saturated water vapour over water liquid at total pressure of 1.5 atm. Vapour pressure of H2O at same temperature is 0.5 atm. What is the solubility of gas 'X' in terms of moles in 10 moles H2O. The slope of the graph is 5000.
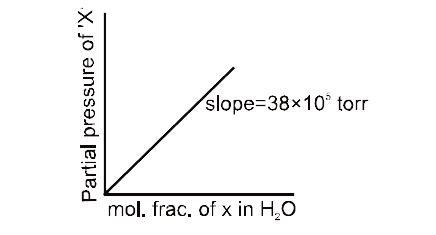
View Solution

