Arrange the following speices according to their bond angle order.
(I) O3
(II) NO−2
(III) FNO
I>II>III
II>I>III
III>II>I
II>III>I
The correct Answer is:A
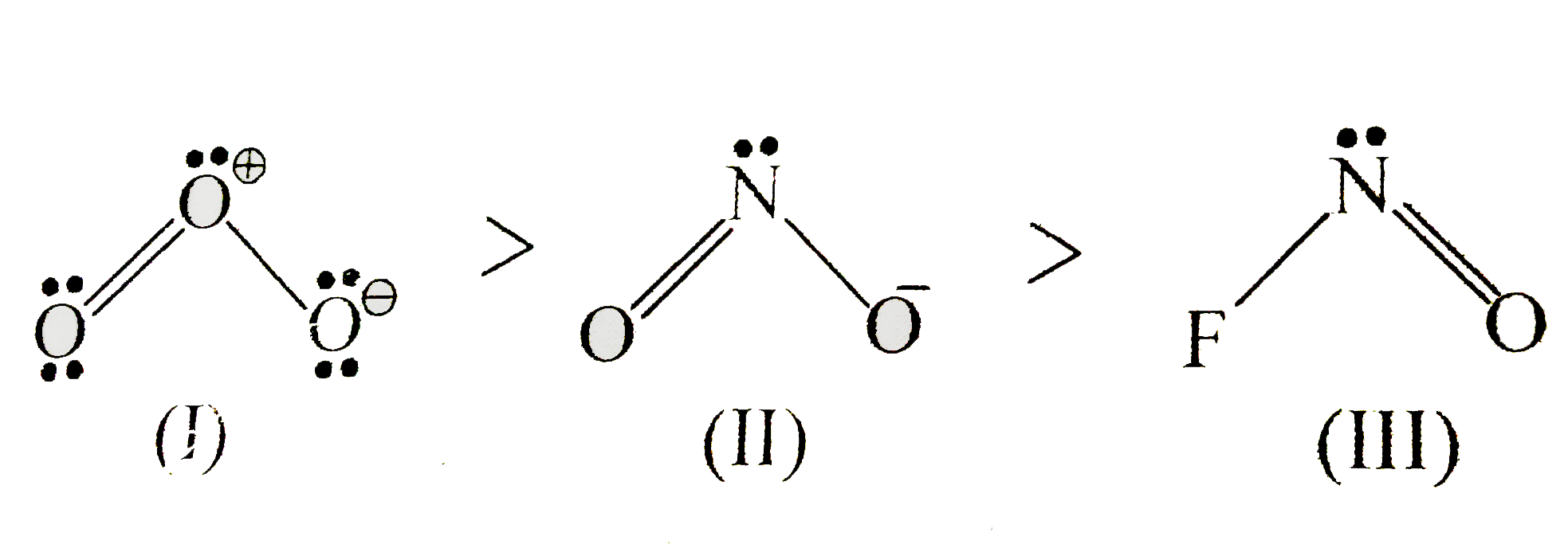
Bond angle α Electronegativity of central atom α1E.N. of side atom
Similar Questions
Arrange nitrogen dioxide molecule (NO_(2)), nitronium ion (NO_(2)^(+)) and nitriteion (NO_(2)^(-)) in incrasing order of bond angle and explain the order.
View SolutionWhich of the following statements are correct with respect to bond order ? (i)Bond order of N_(2) " is " 3.5 (ii) Bond order of O_(2) " is " 2 (iii) Bond order of NO^(+)" is " 3.0 (iv) Bond order of NO is 2.5
View SolutionArrange the following in the order of property indicated for each set: NO_(2), NO_(2)^(+), NO_(2)^(–) (decreasing bond angle)
View SolutionArrange the following in the increasing order order of their bond order : O2,O+2,O−2 and O2−2 :
View SolutionThe correct order of N-O bond length in No, NO−2 and NO−3 will be
View SolutionThe correct order of bond order values amog the following is
(i) NO−2 ii. NO+ iii. NO
iv. NO+2 v. NO−2
View SolutionArrange the following in increasing order of their bond order: O_(2),O_(2)^(+),O_(2)^(-) and O_(2)^(2-)
View SolutionArrange the following in the order of their acidic strength. (i) CH_(3)-NO_(2) (ii) NO_(2) - CH_(2)-NO_(2) (iii) CH_(3) - CH_(2) - NO_(2) (IV) NO_(2) - underset(NO_(2))underset(|)(CH)-NO_(2)
View Solution
