State the following:
Raoult's law in its general term with reference to solution? Henry's law about partial pressure of a gas in mixture.
Text Solution
For answer, sonsult section 5 (Vapour pressure of liquid solutions)
For answer, sonsult section 3 (Solubility)
|
Similar Questions
Knowledge Check
Read the passage given below and answer the following questions :
Raoult's law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton's law of partial pressure states that the total pressure Ptotal over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as:
Ptotal= P1+ P2
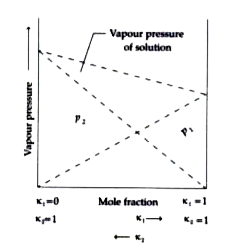
What type of deviation from Raoult's law does the above graph represent ?AFirst positive then negativeBNegative deviationCPositive deviationDFirst negative then positiveRead the passage given below and answer the following questions :
Raoult's law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton's law of partial pressure states that the total pressure Ptotal over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as:
Ptotal= P1+ P2
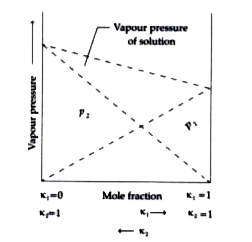
A solution of two liquids boils at a temperature more than the boiling point of either of them. What type of deviation will be shown by the solution formed in terms of Raoult' s law ?ANegative deviationBPositive deviationCFirst positive then negativeDFirst negative then positiveRead the passage given below and answer the following questions :
Raoult's law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton's law of partial pressure states that the total pressure Ptotal over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as:
Ptotal= P1+ P2
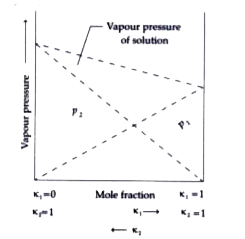
Which of the following aqueous solutions should have the highest boiling point ?A1.0 M NaOHB1.0 M Na2S04C1.0 M NH4NO3D1.0 M KNO3
Raoult's law is for solvent and Henry's law is for solute.
View SolutionRaoult's law is for solvent and Henry's law is for solute.
View SolutionA: Raoult's law applicable for dilute solution only.
R: Henry's law is applicable for solution of gas in liquid.
View SolutionA: Raoult's law applicable for dilute solution only.
R: Henry's law is applicable for solution of gas in liquid.
View SolutionWhich one of the following is an expression of Raoult's law if PA is the partial pressure of the solvent in a solution,P0A is the partial pressure of the pure solvent and if xA and xB are the mole fraction of the solute and the solvent repectively?
View Solution

