A substance C4H10O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8, The structure of the compound is
A
CH3CH2CH2CH2OH
B
CH3CHOHCH2CH3
C
(CH3)3COH
D
CH3CH2−O−CH2CH3
Video Solution
Text Solution
The correct Answer is:2
|
Topper's Solved these Questions
- CARBONYL COMPOUNDSBook:NARAYNAChapter:CARBONYL COMPOUNDSExercise:STATEMENT TYPE
Explore 24 Videos
- CARBONYL COMPOUNDSBook:NARAYNAChapter:CARBONYL COMPOUNDSExercise:LEVEL-IV
Explore 37 Videos
- CARBONYL COMPOUNDSBook:NARAYNAChapter:CARBONYL COMPOUNDSExercise:LEVEL-II(C.W)
Explore 30 Videos
- BENZENEBook:NARAYNAChapter:BENZENEExercise:Level-6
Explore 121 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NARAYNAChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:EXERCISE -4
Explore 54 Videos
Similar Questions
Knowledge Check
A substance C44H16O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8 . The structure of the compound is
ACH3CH2CH2CH2OHBCH3CH(OH)CH2CH3C(CH3)3COHDCH3CH2−O−CH2CH3A substance C4H10O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8, The structure of the compound is
ACH3CH2CH2CH2OHBCH3CHOHCH2CH3C(CH3)3COHDCH3CH2−O−CH2CH3A substance C4H10O yield on oxidation C4H5O which gives oxime and positive iodoform test.
The original substance on treatment with conc. H2SO4 gives C4H8. The structure of compound isA B
B C
C D
D
A compound (X) C4H8O Which gives a 2,4-DNP derivative and a positive iodoform test is:
View SolutionCompound 'X' C4H8O which gives 2,4-DNP derivative and negative iodoform test is :
View SolutionGiven are the isomers of C8H8O2.
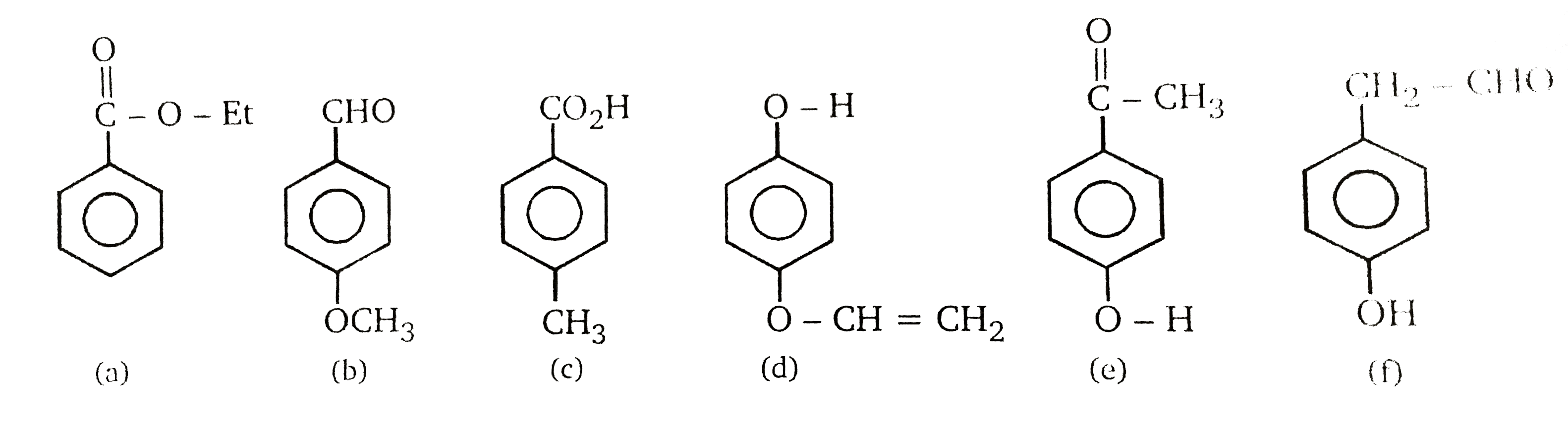
Which isomer gives positive iodoform test?
View SolutionC7H10O reacts with CH3MgBr r to give a compound C8H10O which gives the test with iodoform, than fine out structure of A
View SolutionAn compound (A ) C4H8Cl2 on alkaline hydrolysis to give compound (B ) C4H8O which gives an oxime and positive Tollen's reagent test . What is the structure of (A) ?
View Solution

