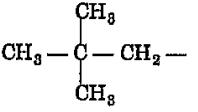
Statement: I : The solubility of aldehydes and ketones in water decreases with increase of size of the alkyl group
Statement - :II: Alkyl groups are eletron releasing groups
Both the statements are true and statement- II is the correct explanation of statement -I.
Both statements are true but statement -II is not the correct explanation of statement -I.
Statement-I is true but statement -II is falsae
Statement -I is false but statement- II is true
The correct Answer is:2
Topper's Solved these Questions
- CARBONYL COMPOUNDSBOOK - NARAYNACHAPTER - CARBONYL COMPOUNDSEXERCISE - LEVEL-IV
37 Videos
- CARBONYL COMPOUNDSBOOK - NARAYNACHAPTER - CARBONYL COMPOUNDSEXERCISE - LEVEL-I(H.W)
19 Videos
- CARBONYL COMPOUNDSBOOK - NARAYNACHAPTER - CARBONYL COMPOUNDSEXERCISE - LEVEL -III
54 Videos
- BENZENEBOOK - NARAYNACHAPTER - BENZENEEXERCISE - Level-6
121 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - NARAYNACHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - EXERCISE -4
54 Videos
Similar Questions
Knowledge Check
Solubility of alcohols decreases with increase in alkyl group because of
Adecrease in hydroge bonding.Bdecrease in van der Waals forces.Cincrease in hydrocarbon part.Ddecrease in polar part.General formula of alkyl group is
AcnH2nBcnH2n+2CcnH2n+1DcnH2n−1In aldehydes and ketones, carbon of carbonyl group is
Asp3 hybridisedBsp2 hybridisedCsp hybridisedDUnhybridised
What is the general formula of alkyl group ?
View SolutionIn aldehydes and ketones, carbon of carbonyl group is
View SolutionStatement-1: Solubility on n-alcohol in water decreases with increse in molecular weight.
Statement-2: The hydropbobic nature of alkyl chain increases.
View SolutionCarbonyl group in aromatic aldehyde and ketone is
View SolutionAssertion : Solubility of alcohols in water decreases with the increase in the molecular mass.
Reason : The increase in the size of the alkyl group increases the polar nature of O−H group.
View Solution