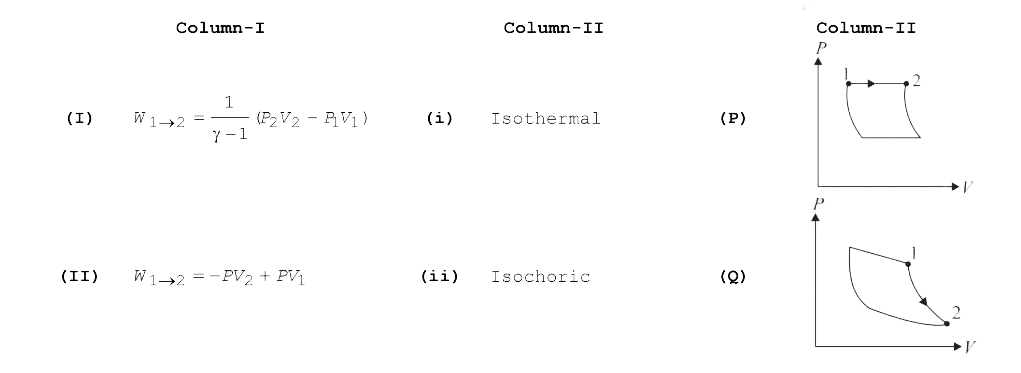
In the graph shown below A-B, B-C , C-D are parts of the carnot cycle operating on an ideal gas.Consider only the path from State 1 to State 2 . W denotes the work in the path. Equations and plots in the table have standard notations γ is the ratio of heat capacities at constant pressure and constant volume. The moles of the gas is 'n'.

For which of the following options dQrevsysT=0 and dQrev(sys)T=0 it taken irreversibly
(I)(iv)(Q)
(IV)(i)(P)
(II)(iii)(R)
None of these
The correct Answer is:A
For adiabatic reversible dQreversible=0 and if taken irreversible dQreversible=0
Hence correct option is (I)(iv)(Q)
Similar Questions
During an adiabatic process of an ideal gas, if P is proportional to 1V1.5, then the ratio of specific heat capacities at constant pressure to that at constant volume for the gas is
View SolutionAn ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding P-V diagrams in column 3 of the table. Consider only the path from state 1 to state 2. W denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic process. Here is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas in n.

Which of the following options is the only correct representation of a process in which ΔU=ΔQ−PΔV?
View Solution
Knowledge Check
In the graph shown below A-B, B-C , C-D are parts of the carnot cycle operating on an ideal gas.Consider only the path from State 1 to State 2 . W denotes the work in the path. Equations and plots in the table have standard notations γ is the ratio of heat capacities at constant pressure and constant volume. The moles of the gas is 'n'.

For which of the following options dQirrev(sys)T>dQrev(sys)TA(I)(iv)(Q)B(IV)(i)(P)C(II)(iii)(R)DNone of theseIn the graph shown below A-B, B-C , C-D are parts of the carnot cycle operating on an ideal gas.Consider only the path from State 1 to State 2 . W denotes the work in the path. Equations and plots in the table have standard notations γ is the ratio of heat capacities at constant pressure and constant volume. The moles of the gas is 'n'.

For which of the following options dQrev(sys)T>0 and simultaneously dQirrev(sys)T>0 If taken irreversiblyA(I)(iv)(Q)B(IV)(i)(P)C(II)(iii)(R)DNone of theseAn ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding P-V diagrams in column 3 of the table. Consider only the path from state 1 to state 2. W denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic process. Here is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas in n.


Which of the following options is the only correct representation of a process in which ΔU=ΔQ−PΔV?A(II) (iv) (R)B(III) (iii) (P)C(II) (iii) (P)D(II) (iii) (S)
For one mole of an ideal gas(CpandCv are molar heat capacities at constant presure and constant volume respectively)
View SolutionThe ratio of the molar heat capacities of a diatomic gas at constant pressure to that at constant volume is
View SolutionCvandCp denote the molar specific heat capacities of a gas at constant volume and constant pressure, respectively. Then
View SolutionCvandCp denote the molar specific heat capacities of a gas at costant volume and constant pressure, respectively. Then
View SolutionFor an ideal monoatomic gas, the universal gas constant R is n times the molar heat capacity a constant pressure Cp.Here n is
View Solution

