Choose the correct option(s)
A
At low pressure (nearly 1 atm), compressibility factor for H2 gas is greater than 1 at 273 K.
B
Compressibility factor for a vander Waal's gas at its critical condition is less than 1
C
Boyle's temperature of a gas is lesser than its critical temperature
D
vander Waal's constant 'a' for NH3 is greater than that of CH4
Text Solution
The correct Answer is:A,B,C
(A) For H2 at 273 K, Z>1
(B)ZC=38
( C)Tb=aRbTc=8a27Rb=827×Tb
Tb>Tc
|
Similar Questions
Knowledge Check
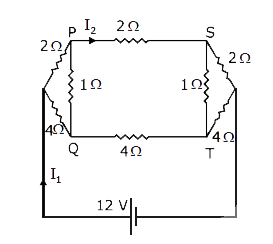
For the resistance network shown in the figure, choose the correct options (s)
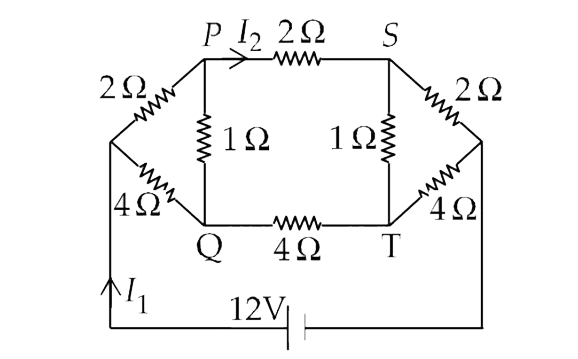 AThe current through PQ is zero.BI1=3ACThe potential at S is less than that at Q.DI2=2A
AThe current through PQ is zero.BI1=3ACThe potential at S is less than that at Q.DI2=2A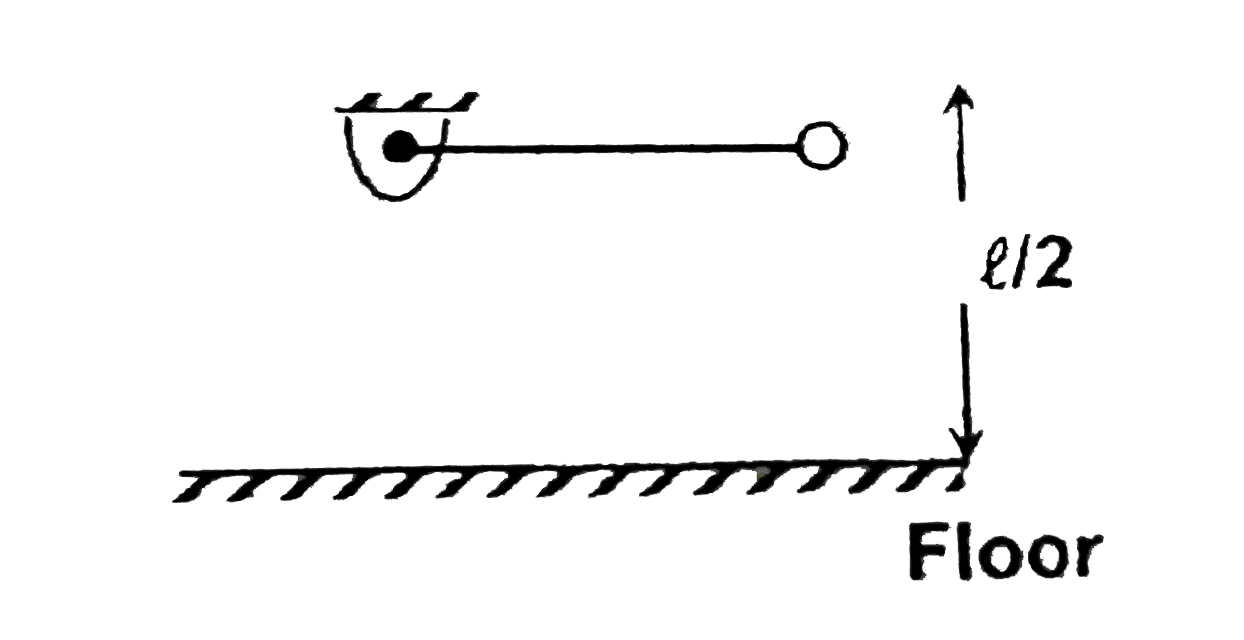
A bob of mass m connected to the end of an inextensible string of length l, is released from position shown in figure. If impacts of bob with smooth floors is perfectly inelastic. Choose the correct options (s)AThe maximum height reached by the bob during subsequency motion is l32BMaximum height reached by the bob during subsequency motion is zero.CEnergy loss from the time duration bob is released to the time it reaches at its maximum height during subsequent motion is 3mgl8DEnergy loss from the time duration bob is released to the time it reaches to the time it reaches at its maximum height during subsequency motion is 15mgl32If a projectile explods in mid air then just before and just after explosion, choose correct option(s)
ACronservation of momentum is applicableBPath of the centre of mass of the projectile must be same original parabola till any one part of it, his the groundCEnergy relased druing explosion is equal to increase in kinetic energy of the projectileDConservaton of momentum is not applicable.