
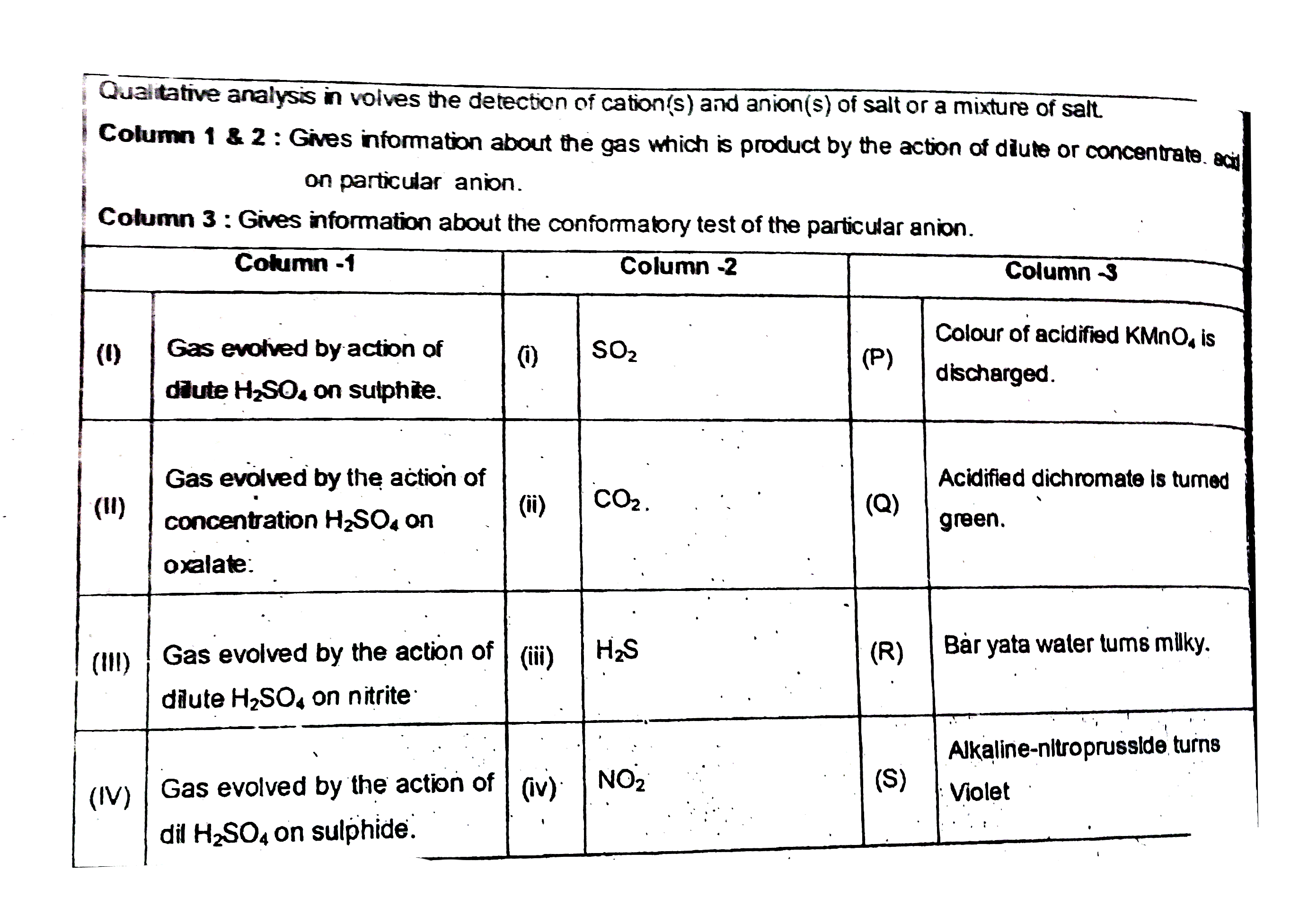
The only correct combination is :
(I)(iii)(R)
(II)(i)(S)
(III)(ii)(P)
(IV)(iv)(Q)
The correct Answer is:A
(I)First line of Balmer series
n1=2 n2=3
ΔP.E=27.2×(Z)2[1n21−1n22]
=27.2×(1)2[14−19]=27.2×536=3.78ev
ΔE=13.6×(1)2[14−19]=1.88ev
¯v=R×(1)2[14−19]=5R36
λ=365R
Angular momentum=3h2π−2h2π=h2π
(II)Third line of paschen series of He+ ion
n1=3 n2=6
ΔP.E=27.2×(2)2[19−136]=9.06ev
ΔE=13.6×(2)2[19−136]=4.54ev
¯v=R×(2)2[19−136]=R3
λ=3R
Angular momentum=6h2π−3h2π=3h2π
(III)Lyman series limit for Li+2 ion
n1=1 n2=∞
ΔP.E=27.2×(3)2[112−1∞2]=27.2×9=244.8ev
ΔE=13.6×(9)[112−1∞2]=122.4ev
¯v=R×(3)[112−1∞2]=9R
(IV)2nd line of Lyman series for He+ ion
n1=1 n2=3
ΔP.E=27.2×(2)2[11−19]=96.71ev
ΔE=13.6×(2)2[11−19]=48.4ev
¯v=R×(2)2[11−19]=32R9,λ=932R
Angular momentum=3h2π−h2π=2h2π=hπ
Topper's Solved these Questions
Similar Questions
The only incorrect combination is-
View SolutionMatch the following :
Answer the following qeustions by appropriately matching the information given in the three columns of the following table.
Columns, 1,2 and 3 containing starting materials, reaction conditions and type of reactions, respectively.
The only correct combination in which the reaction proceeds through radical mechanism is :
View Solution
Knowledge Check
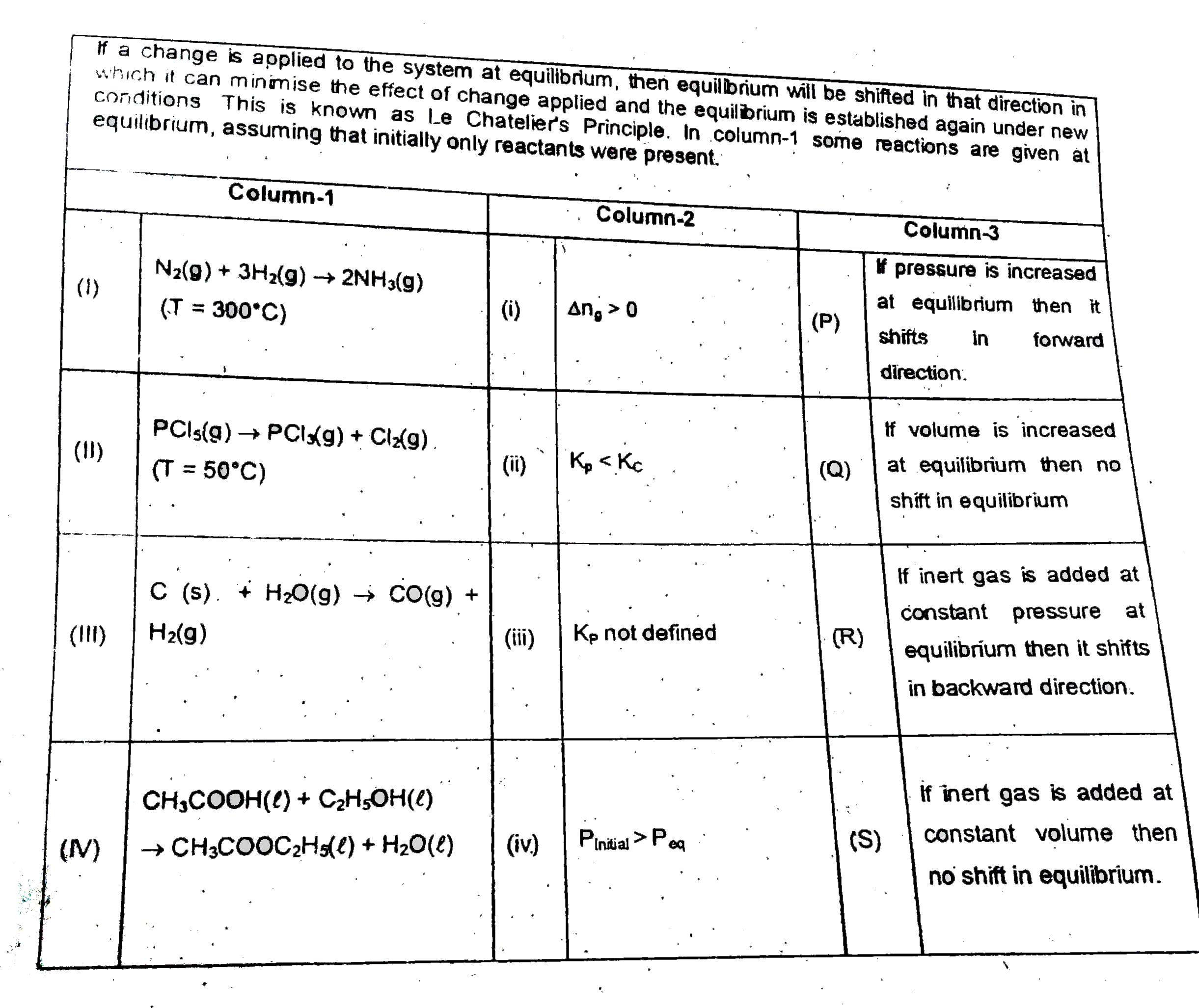
The only incorrect combination is :A(I)(ii)(P)B(I)(iv)(R)C(III)(i)(S)D(IV)(ii)(Q)
The only incorrect combination is :A(III)(ii)(P)B(I)(iv)(Q)C(IV)(iv)(Q)D(II)(iii)(Q)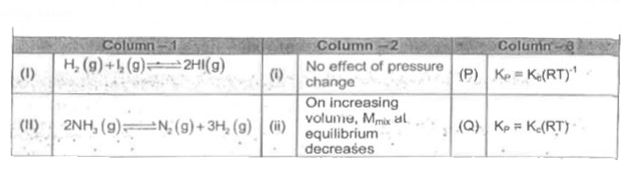

The only incorrect combination isA(I)(iii)(R)B(IV)(i)(Q)C(I)(i)(S)D(IV)(iii)(S)
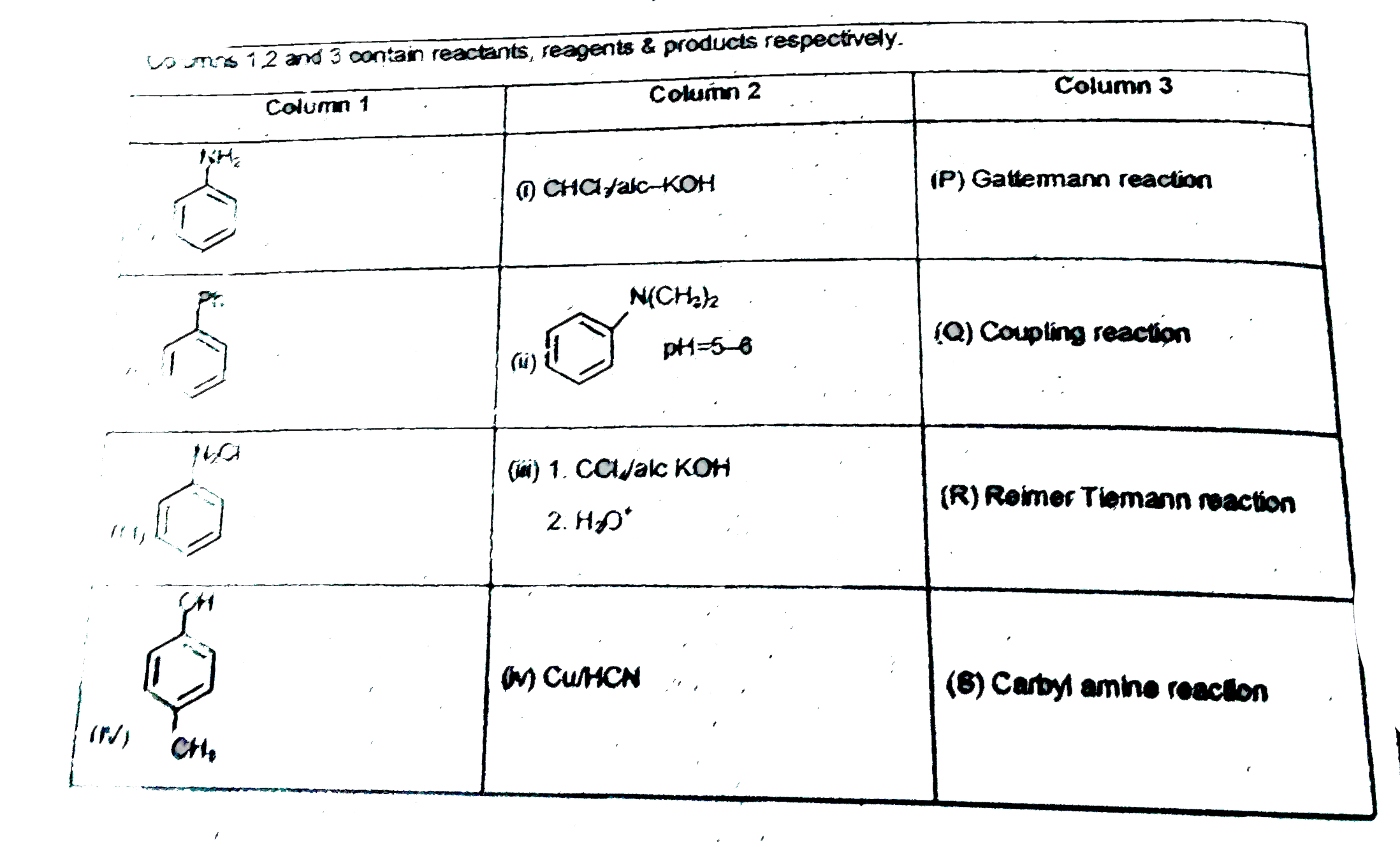
The only correct combination in which carboxylic acid is formed.
View Solution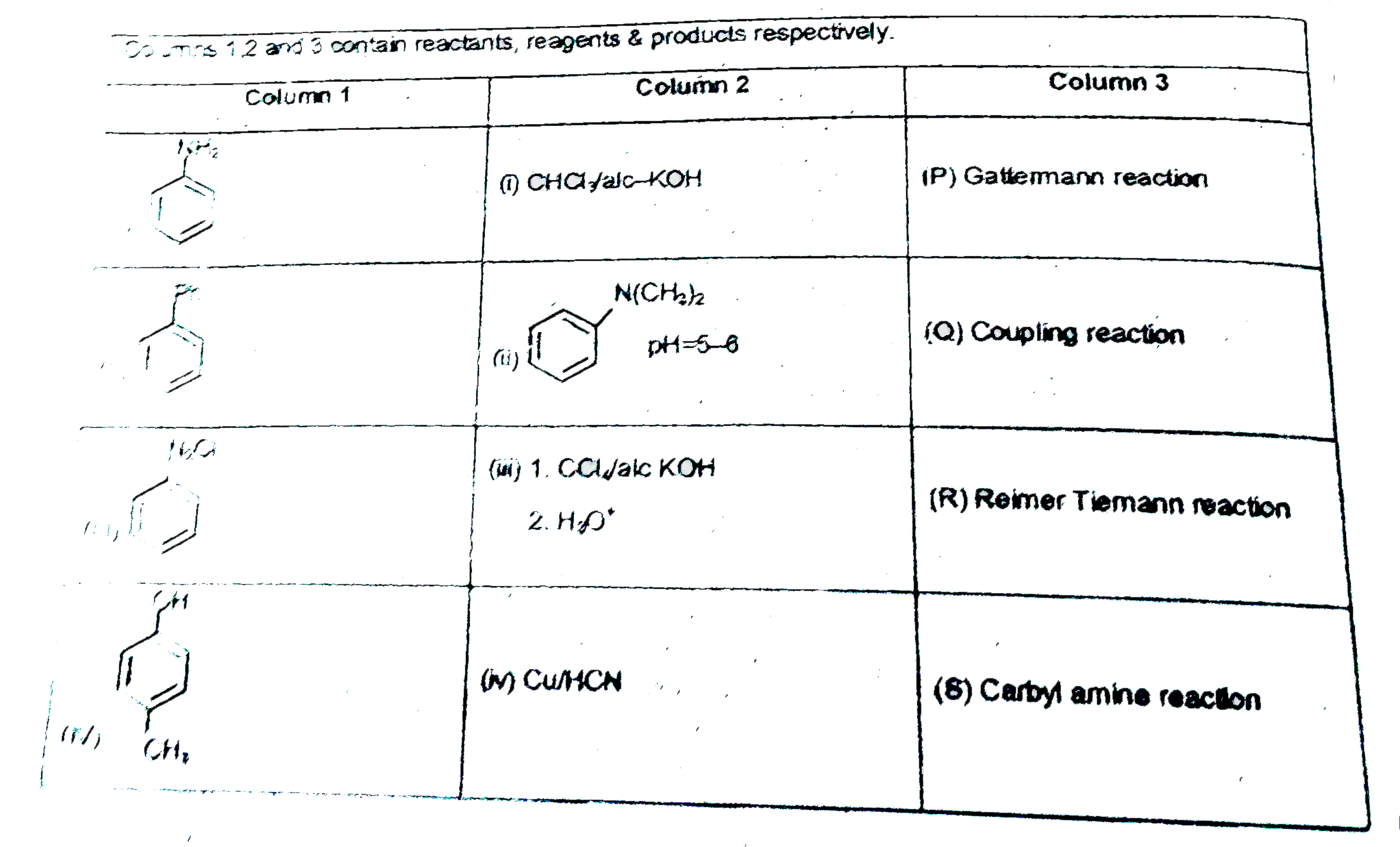
The only correct combination in which isocyanide is formed
View Solution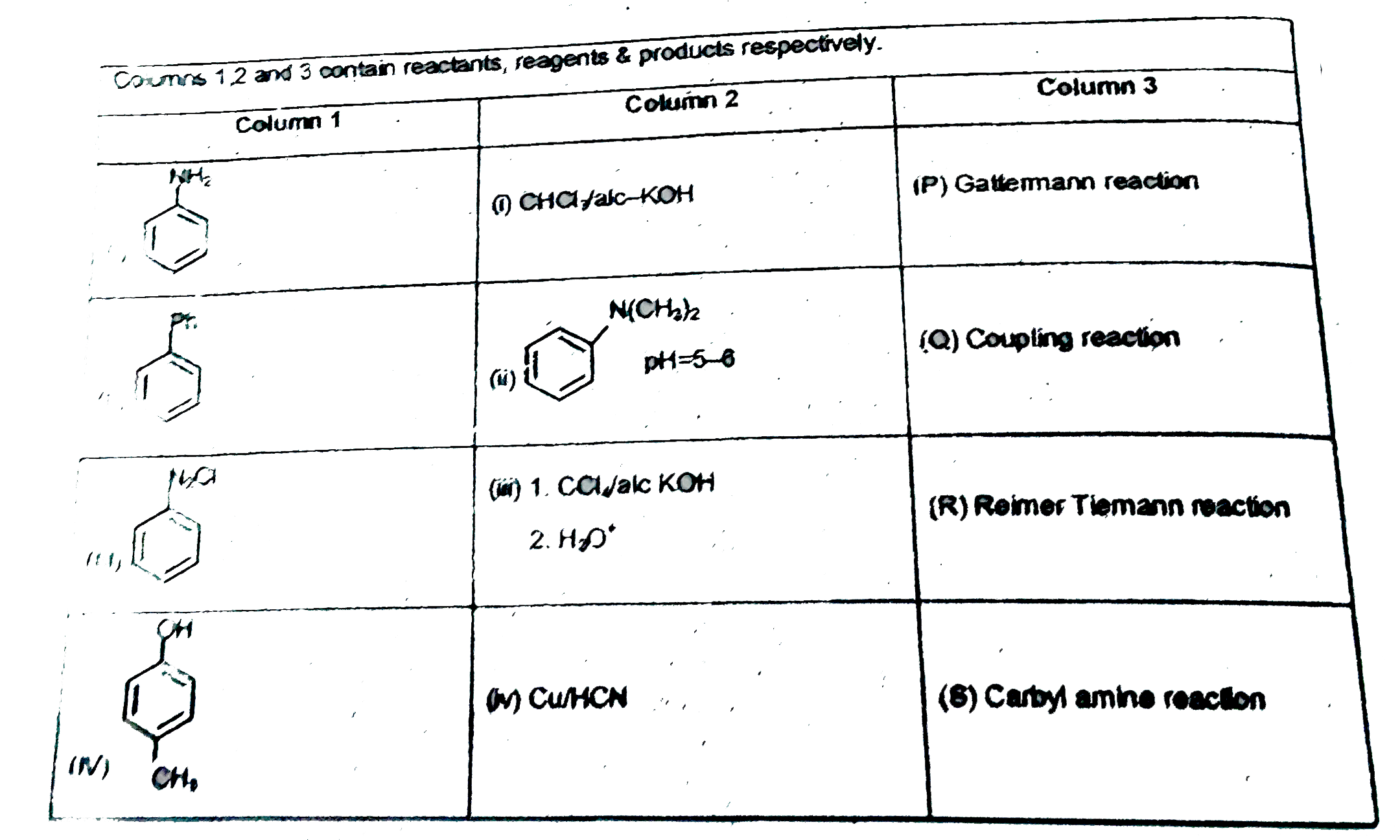
The only correct combination in which Azo dye is formed.
View Solution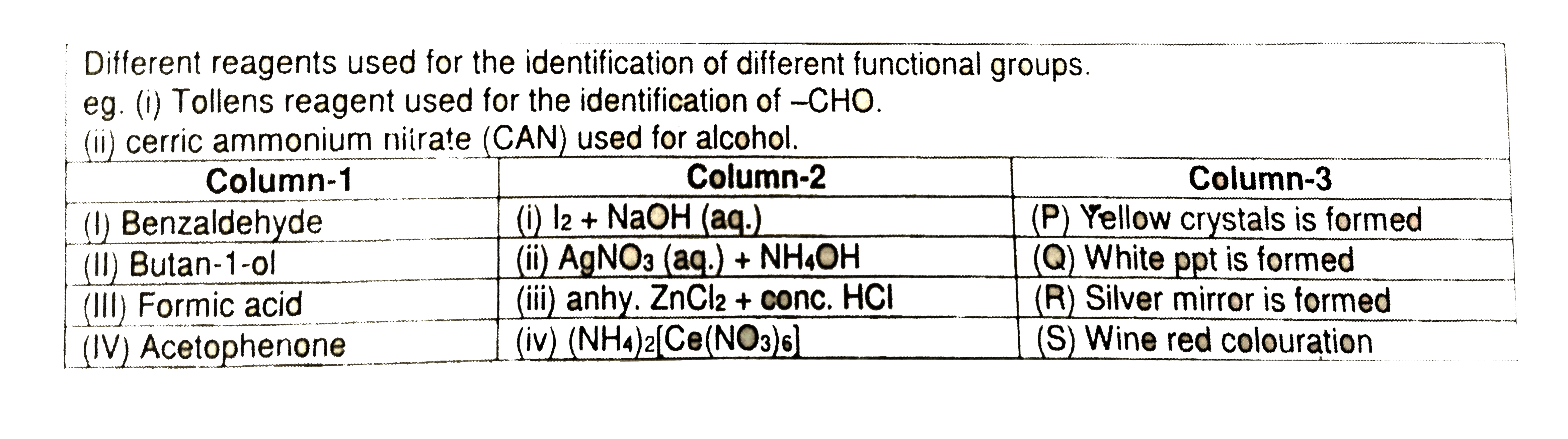
The only correct combination in which the reaction does not proceed through redox mechanism.
View Solution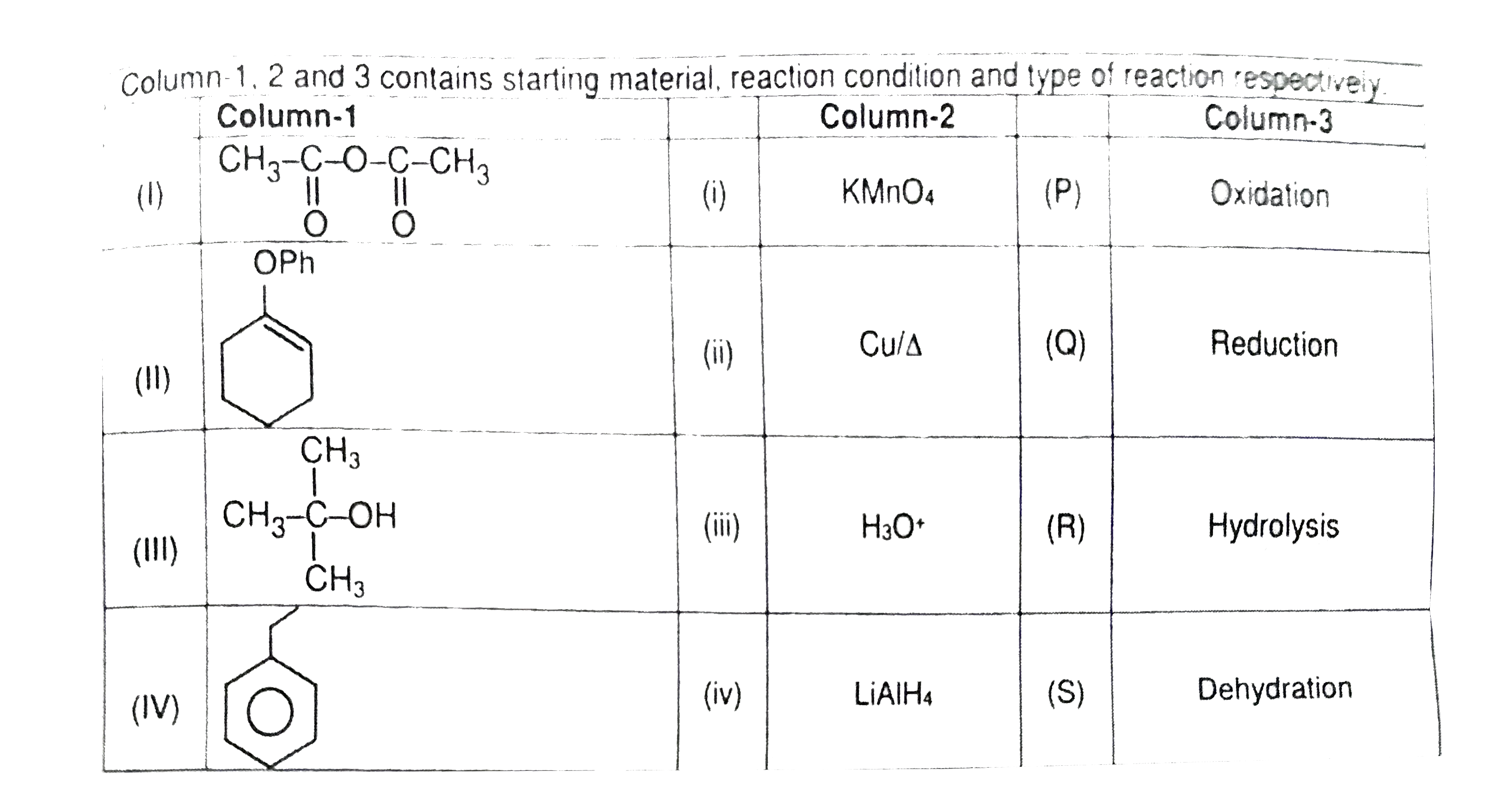
The only correct combination in which product gives position test with sodium bicarbonate is -
View Solution

