How many of the following statements are correct ?
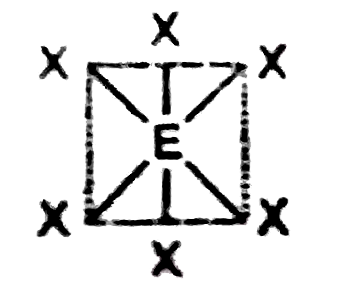
(i)Among sp3d,sp3d2andsp3d3 hybridisation, the maximum number of 90∘ angles between bond pair bond pair of electrons is observed in sp3d2 hybridisation .
(ii)Among SF4,CF4andXeF4, interatomic bond angle of 109∘28' is observed only in SF4.
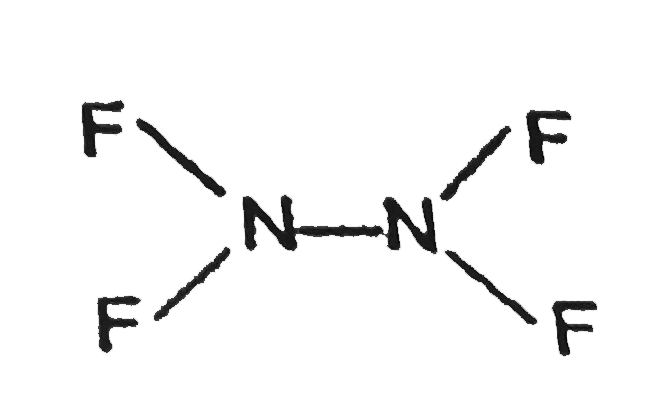
(iii)Among N2F4,N2F2,andN2 , the largest N-N bond length is found in N2F4.
(iv)Among NF3,NO−3,BF3,H3O+andNH3,[NH3,H3O+]and[BF3,NO−3] are isostructural pairs.
(v)Among NH3,PH3,AsH3,andSbH3,SbH3 has smallest bond angle.
(vi)Among SbCl3,SbBr3,andSbl3,Sbl3 has the largest bond angle.
Text Solution
The correct Answer is:5
(i)Bond pair-bond pair bond angles in sp3d2 are 12.
(ii)109∘28' bond angle is observed only in CF4 (tetrahedral geometry)
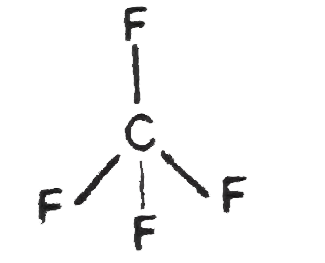
(iii)N-=N(~~1.1CÅ)ltFN=NF(~~1.21Å)lt
(1.49±0.004)
(iv)BF3 and NO−3 ,both trigonal planar , NF3 and H3O+,both trigonal pyramidal.
(v)Order is NH3>PH3>AsH3>SbH3106∘6 93.8∘ 91.83∘ 91.3∘
(v)Order is Sbl3>SbBr3>SbCl399∘ 98.2∘ 97.1∘
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
sp3d2 hybridisation is present in
A[CoF6]−3B[Ni(CO)4]C[Co(NH3)6]+2D[Ni(CN)4]−2A molecule is formed by sp3d2 hybridisation. Bond angle in it is :
A90∘B109∘28C90∘and120∘D120∘.The maximum number of 180∘ angle possible between X-M-X bond for compounds with sp3d2 and sp3d hybridisation respectively are
A3,3B3,1C1,3D3,0
Which d - orbitals are involved in sp3d2 hybridisation in SF6?
View Solutionsp3d hybridisation | sp3d2 hybridisation | sp3d3 hybridisation
View Solutionsp3d hybridisation | sp3d2 hybridisation | sp3d3 hybridisation
View SolutionN−N bond length is minimum in
View SolutionThe correct order of bond angles between bond pair−bond pair of electrons ( biggest first ) in +NO2,H2O,CCl2 and BF3 is :
View Solution

