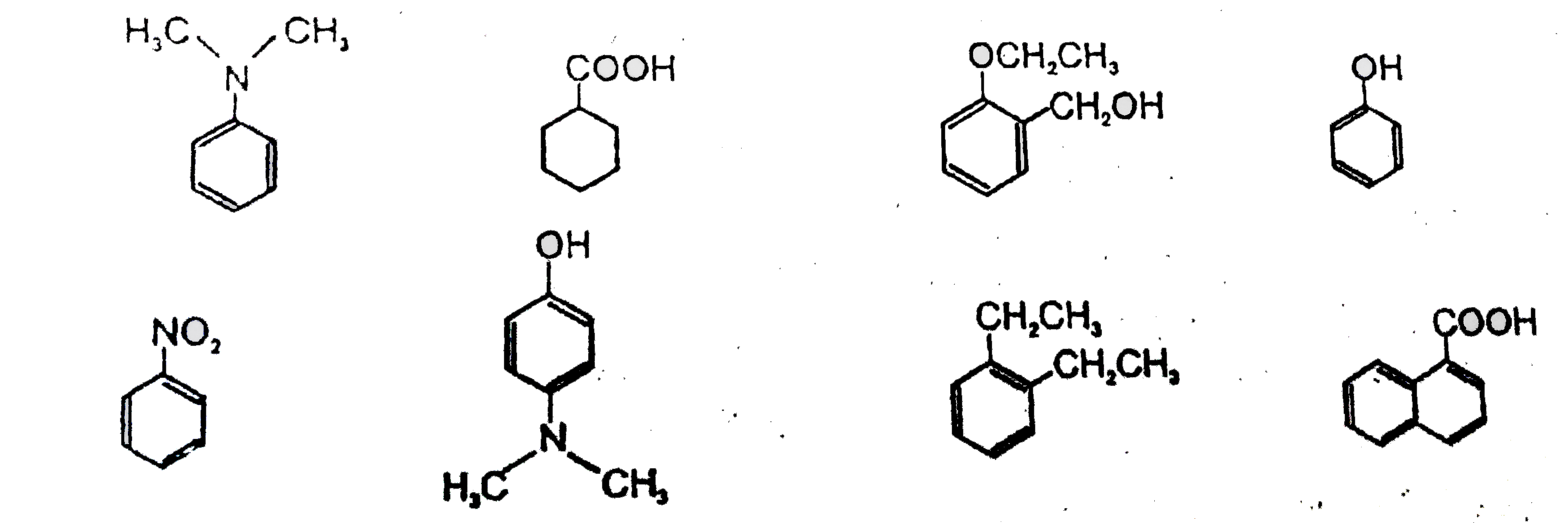
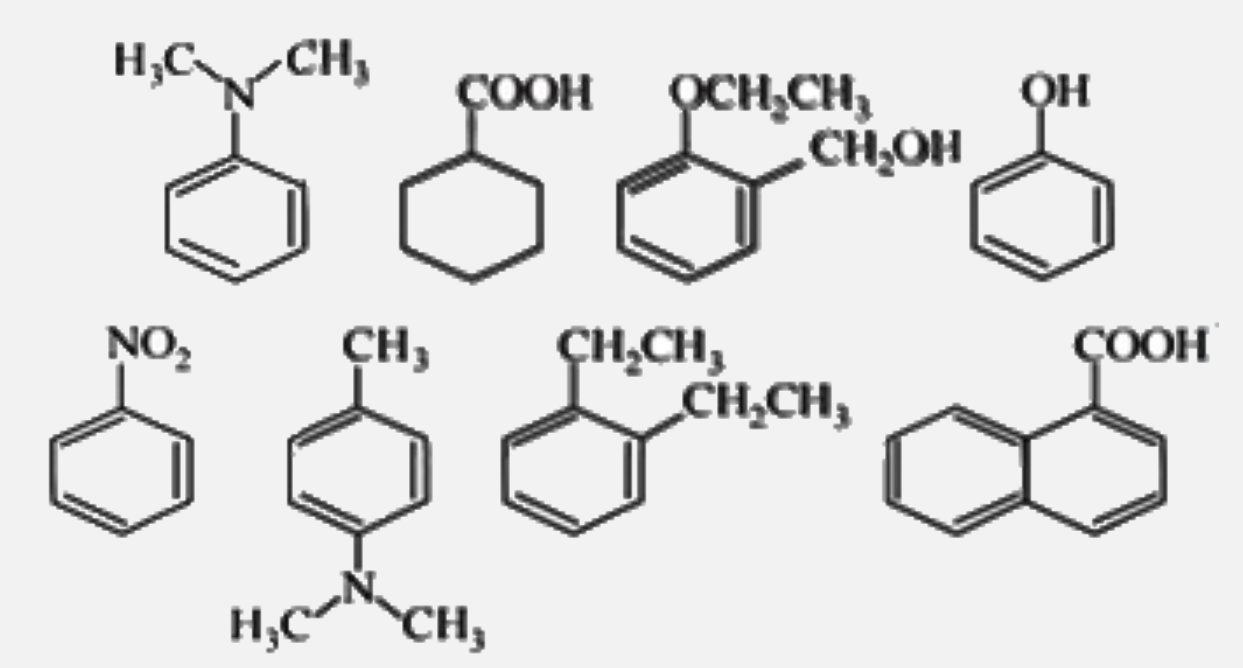
Amongst the following the total number of the compounds i.e. oxide soluble in aqueous NaOH is :
Ag2O,Al2O3,ZnO,SnO,PbO,CuO,BeO,BaO,CaO
Text Solution
The correct Answer is:5
BeO(s)+2NaOH(aq)→Na2BeO2(aq)+H2O
Al2O3(s)+2NaOH(aq)→2NaAlO2(aq)+H2O
ZnO(s)+2NaOH(aq)→Na2ZnO2(aq)+H2O
SnO(s)+2NaOH(aq)→Na2SnO2(aq)+H2O
PbO(s)+2NaOH(aq)→Na2PbO2(aq)+H2O
|
Similar Questions
Knowledge Check
Amongest the following, the total number of compound soluble in aqueous NaOH is :
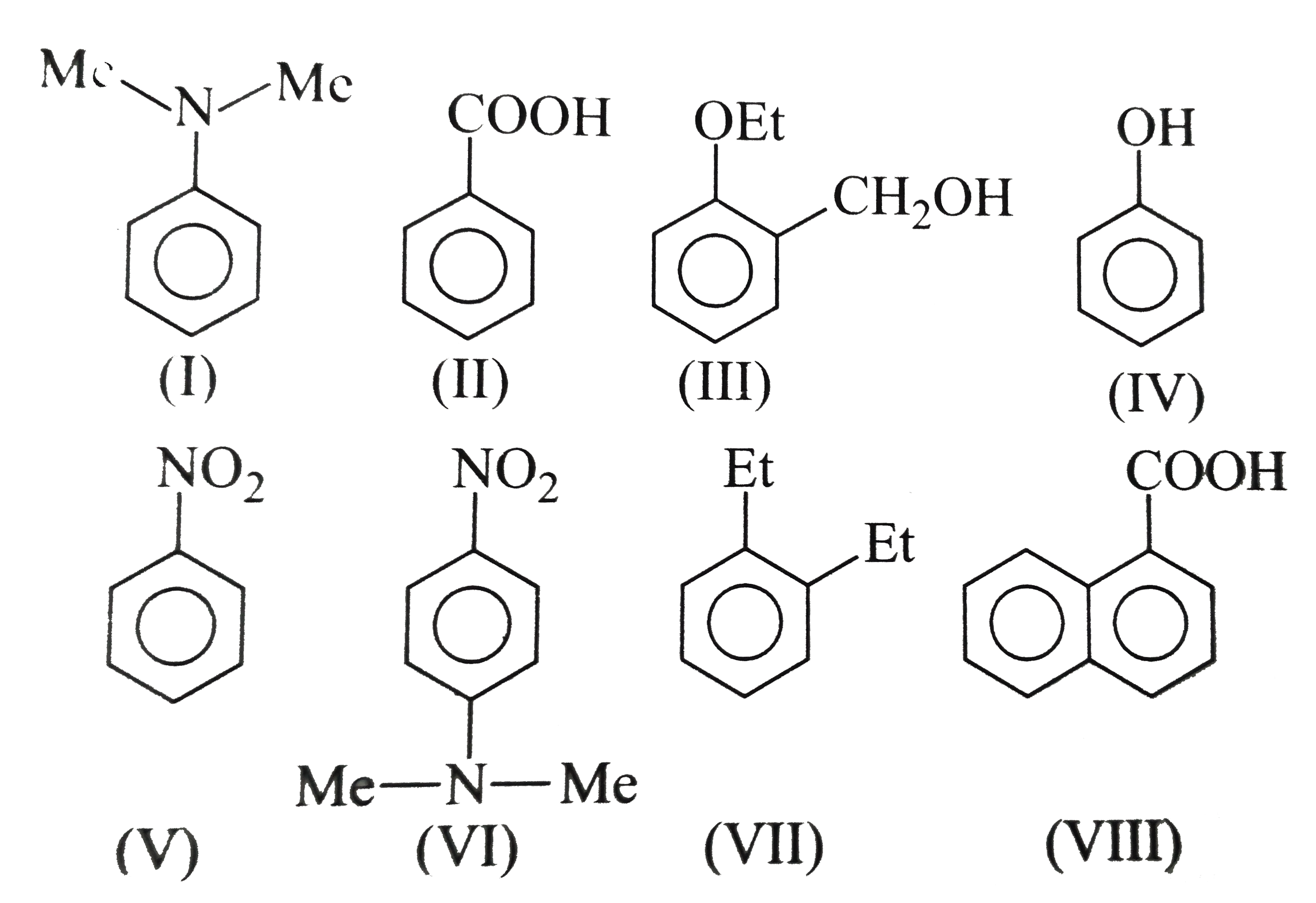 .A(V)B(VI)C(VII)D(VIII)
.A(V)B(VI)C(VII)D(VIII)
Among the following, the total number of compound soluble in aqueous NaOH is :
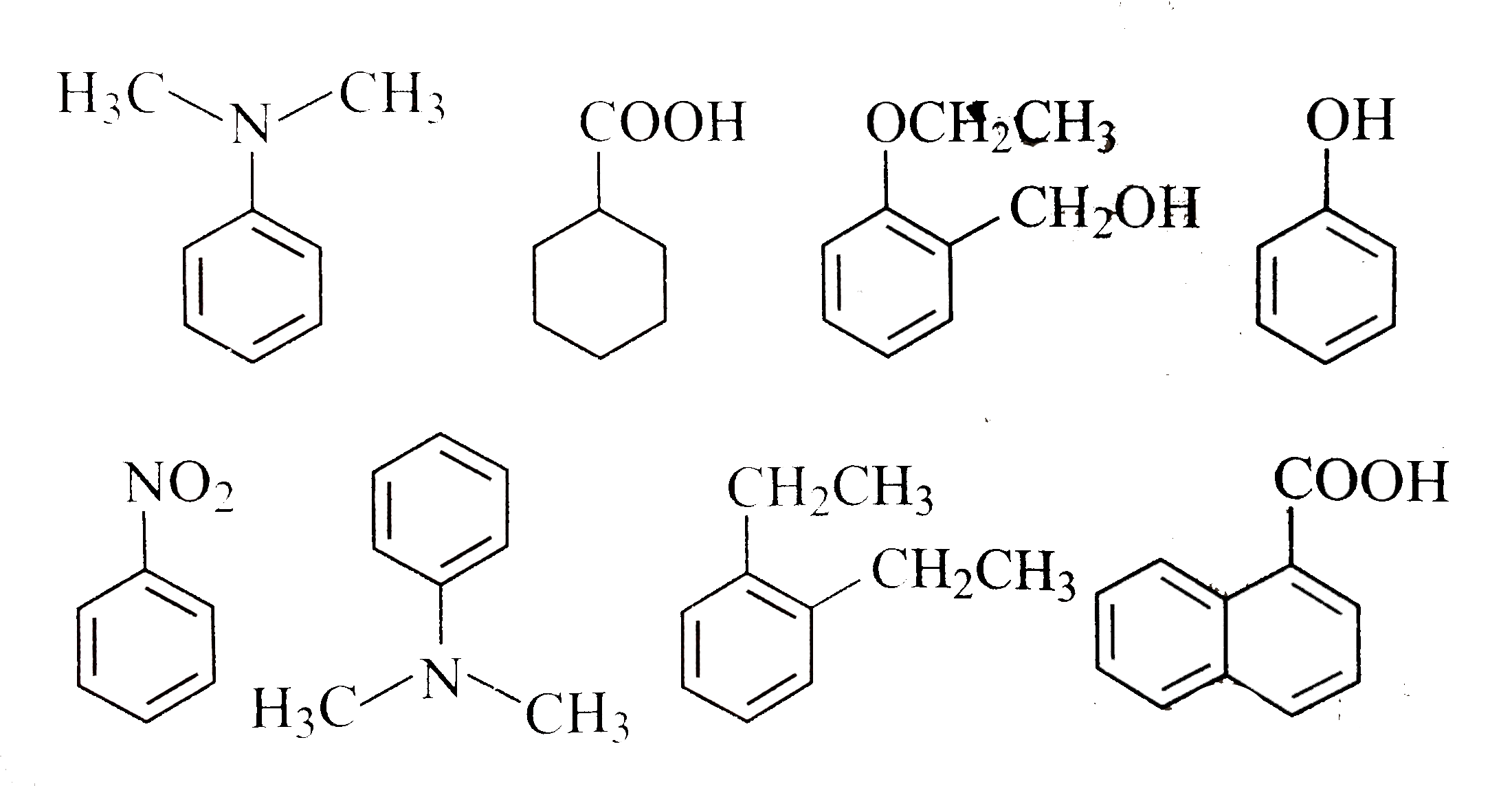 .
.
View SolutionAmongst the following, the number of compounds soluble in aqueous NaOH is
?
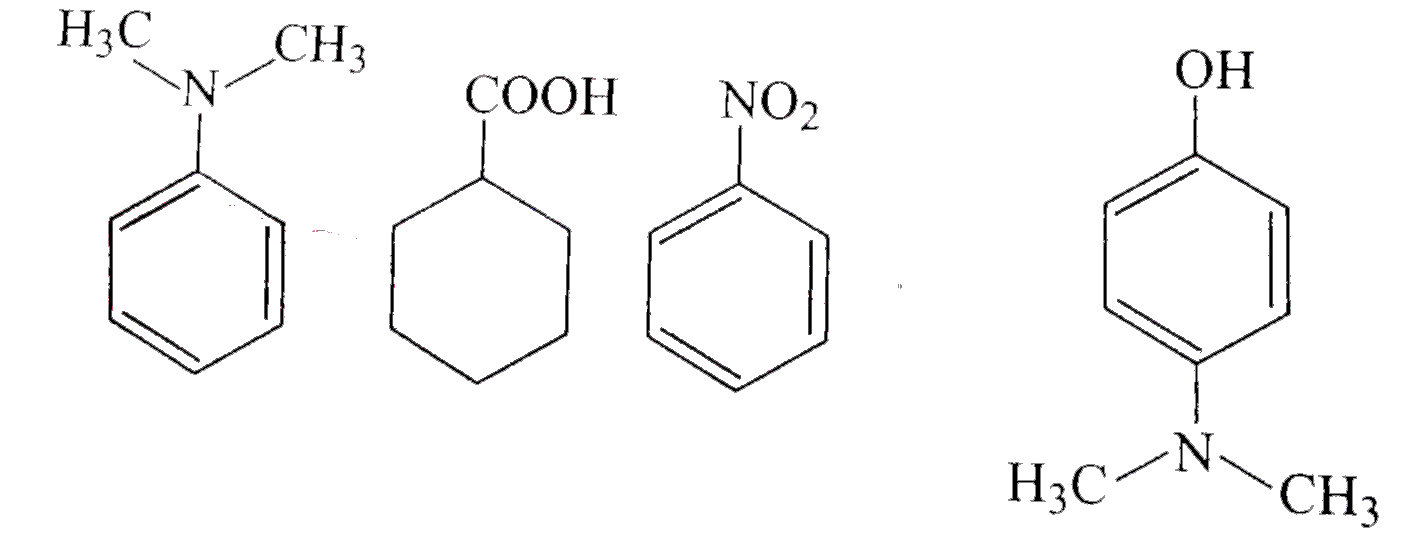 ,
, 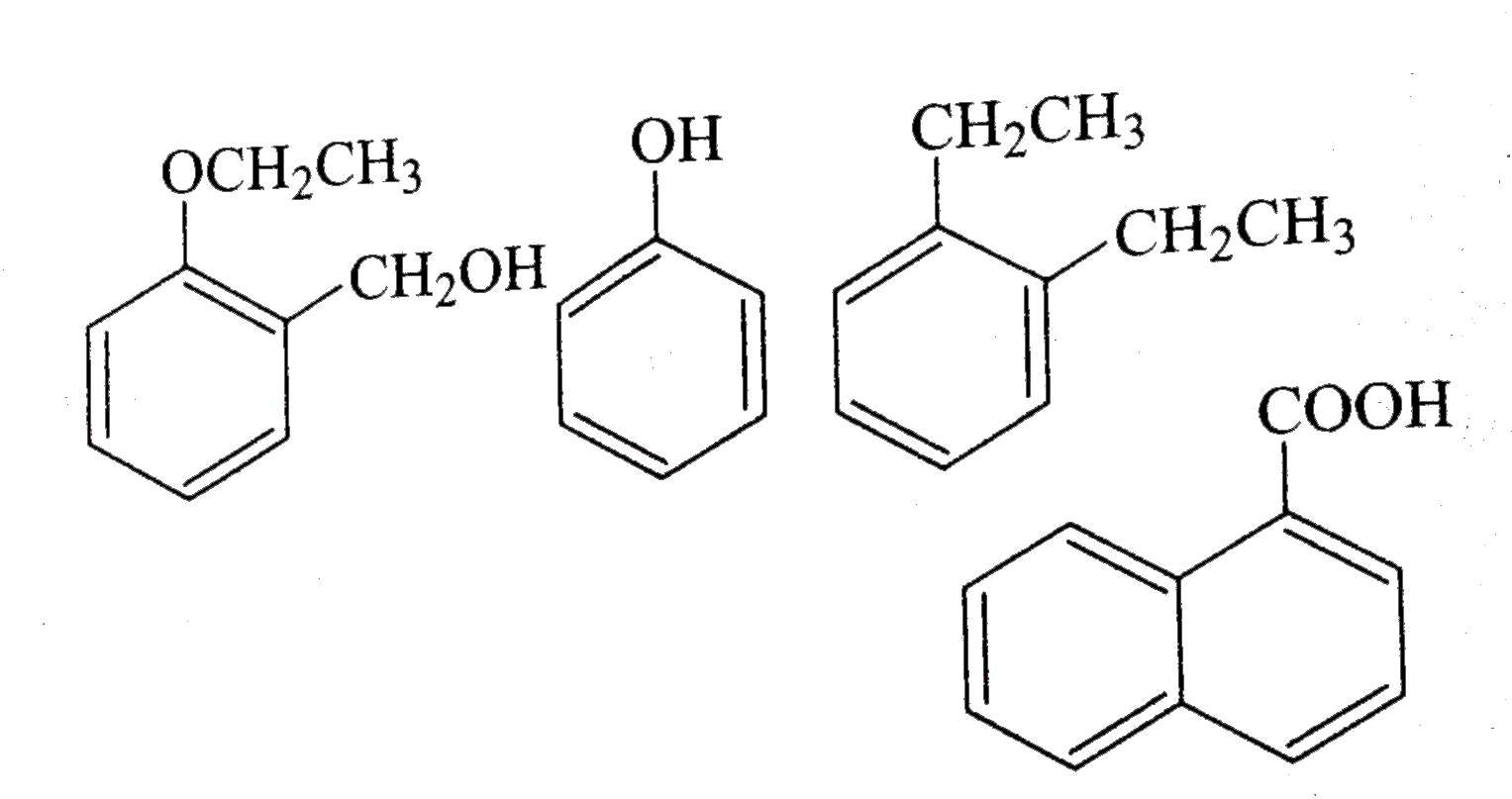
View SolutionAmongst the following, the following , the total number of amphoteric oxides is CO,SnO,PbO,CO2,SiO2,GeO2,SnO2,PbO2
View SolutionFind the total number of amphoteric compound from following BeO,Be(OH)2,BaO,Sr(OH)2
View SolutionHow many total number of compounds are only acidic in aqueous medium -
NA2O,Cl2O7,B2O2,Al2O3,ZnO,Cs2O,BaO,SO3
View SolutionSum of total number of amphoteric and neutral oxides among the folllowing is :
CO,NO,Al2O3,PbO2,CaO,SnO2,ZnO
View SolutionChoose the amphoteric oxides amongst the following:
Na2O,ZnO,Al2O3,CO2,H2O
View Solution