Which of the following reagents can be used to distinguish the SO2 and CO2 ?
A
Lime water
B
Zinc nitroprusside paste in water
C
Potassium iodate and starch
D
Acidified potassium dichromate solution
Text Solution
The correct Answer is:B,C,D
(B)Red colour compound of unknown composition is formed when moist SO2 is brought in contact with Zn[Fe(CN)5NO] (salmon colour) paste in water.No colour change with CO2.
(C )5SO2+2IO−3+4H2O→I2+5SO2−4+8H+,I2+ starch → blue colour.
No colour change observed with CO2.
(D)3SO2+Cr2O2−7+2H+→2Cr3+(green solution) +3SO2−4+H2O
No colour change is observed with CO2
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following reagents can be used to distinguish between SO2andCO2?
ALime waterBBaCl2 solutionCH2O2+BaCl2 solution+dil. HClDAcidified dichromate paperWhich of the following reacgents can be used to distinguish between SO2 and CO2?
ALime waterBZine nitropruside paste in waterCPotasium iodate and strachDAcidfied potessium dichromate of aqueousWhich of the following reagents will be useful to distinguish between
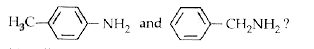 ADilute HCIBC6H5SO2ClandOH−/H2OCHONO then β-naphtolDAgNO3 in H2O
ADilute HCIBC6H5SO2ClandOH−/H2OCHONO then β-naphtolDAgNO3 in H2O
Which of the following reagents can be used to distinguish between a sulphite and a sulphate in solution?
View SolutionWhich of the following reagents can be used to distinguish between propene and propyne?
View SolutionWhich of the following reagents can be used to distinguish a phenol and an alcohol?
View SolutionWhich of the following reagents can be used to distinguish a phenol and an alcohol?
View SolutionWhich of the following reagents cannot be used to distinguish between phenol and benzyl alcohol ?
View Solution

