A student was given a sample of colourless solution containing three cations and was asked to identify the cations.Student carried out a series of reactions as given below.
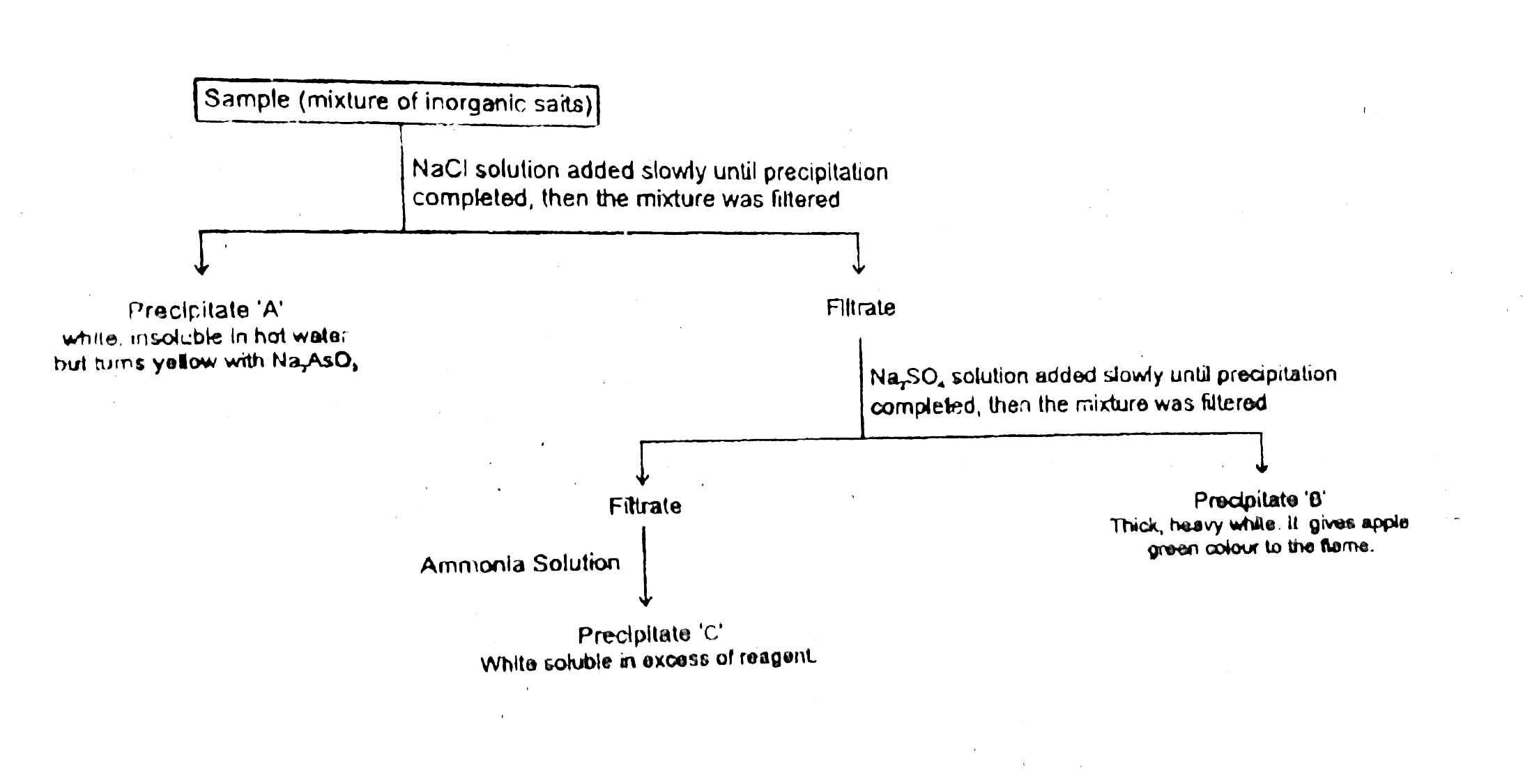
White precipitate 'A' is not soluble in:
NH3
2M HCl
KCN
Na2S2O3
The correct Answer is:B
(A)AgCl+2NH3→[Ag(NH3)2]Cl soluble complex.
(B)2M HCl is group reagent of 1st group but in concentrated HCl AgCl dissolves forming [AgCl2]− complex
(C )AgCl+2CN−→[Ag(CN)2]−(soluble complex) +Cl−
(D)AgCl+2S2O2−3→[Ag(S2O3)2]3−(soluble complex) +Cl−
Topper's Solved these Questions
Similar Questions
A red solid is insoluble in water.However it becomes soluble if some KI is added to water.Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube.The red solid is :
View SolutionSodium (Na=23) crystallizes in bcc arrangement with the interfacial separation between the atoms at the edge 53.6±. The density of sodium crystal is :
View SolutionWhen two colourless solutions are mixed, it results a white precipitate. The two solutions are of
View SolutionA student was given a sample of colourless solution contaning three cations and was asked to identify the cations: student carried out a series of reactions as given below. Precipitates A,B and C are respectively:
View SolutionA student was given a sample of colourless solution contaning three cations and was asked to identify the cations: student carried out a series of reactions as given below. White precipitate A is not soluble in:
View SolutionA student was given a sample of colourless solution contaning three cations and was asked to identify the cations: student carried out a series of reactions as given below. Which of the following statement is correct?
View SolutionWhen a student added a few drops of barium chloride solution to sodium sulphate solution, he obtained a white precipitate instantly. Which of the following type of chemical reaction has been carried out by the student ?
View SolutionA student was given a sample of colourless solution containing three cations and was asked to identify the cations.Student carried out a series of reactions as given below.
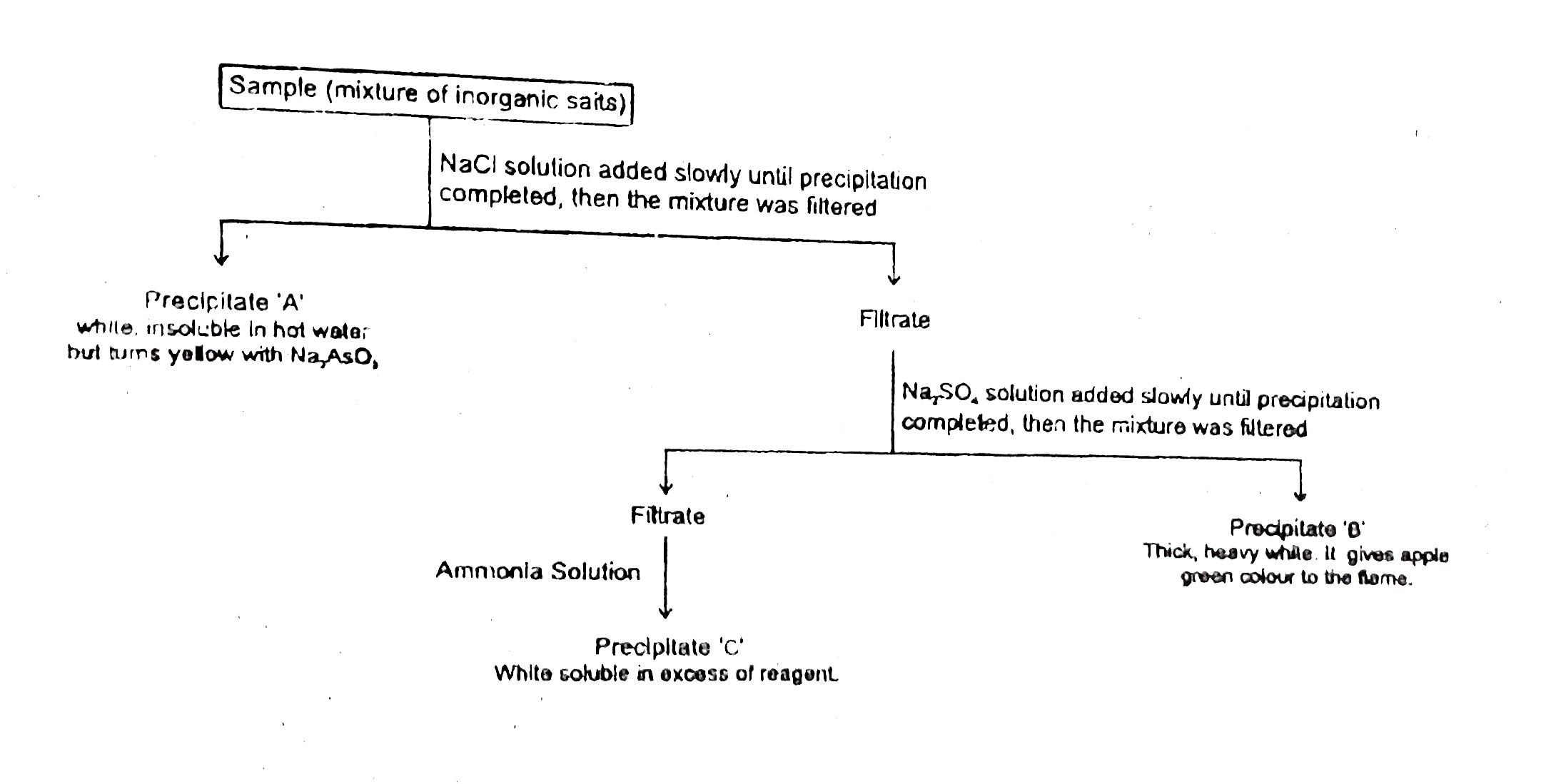
Precipitates 'A','B' and 'C' are respectively :
View SolutionA student was given a sample of colourless solution containing three cations and was asked to identify the cations.Student carried out a series of reactions as given below.

Which of the following statements is correct ?
View SolutionA student was given a sample of colourless solution containing three cations and was asked to identify the cations. Student carried out a series of reactions as given below.
Precipitates 'A','B' and 'C' are respectively:

View SolutionA student was given a sample of colourless solution containing three cations and was asked to identify the cations. Student carried out a series of reactions as given below.
White precipitate 'A' is not soluble in:

View SolutionA student was given a sample of colourless solution containing three cations and was asked to identify the cations. Student carried out a series of reactions as given below.
Which of the following statements is correct ?

View SolutionA solution containing a group-IV cation gives a precipitate on passing, H2S. A solution of this precipitate in dil. HCl produces a white precipitate with NaOH solution and bluish-white prcipitate with basic potassium ferrocyanide. The cation is :
View SolutionA solution containing a group-IV cation gives a precipitate on passing, H_(2)S . A solution of this precipitate in dil. HCl produces a white precipitate with NaOH solution and bluish-white prcipitate with basic potassium ferrocyanide. The cation is :
View SolutionThe ferrous ion in a given sample is detected by the formation of a white precipitate on the addition of a potassium ferrocyanide solution to it. The precipitate has the constitutional formula:
View Solution

