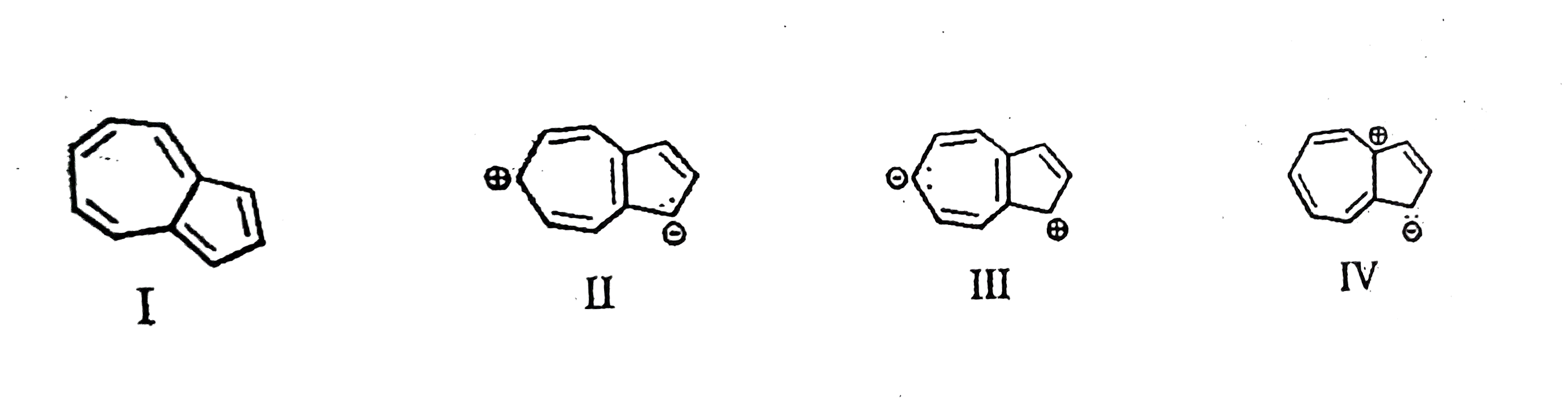
The most stable and the least stable resonating structures are respectively
A
I and IV
B
I and III
C
II and III
D
III and II
Text Solution
The correct Answer is:C
In structure II, both the rings are aromatic hence II is most stable structure but in structure III, one ring contain 8π electron hence antiaromatic , hence structure III is least stable structure.
|
Similar Questions
Knowledge Check
The most stable resonating structure is
AH2N−⊕CH−CH=CH−OCH3BH2⊕N=CH−CH=CH−OCH3C DNH2−⊕CH−ɵCH−CH=⊕OCH3
DNH2−⊕CH−ɵCH−CH=⊕OCH3The least and most stable resonating structure respectively are :
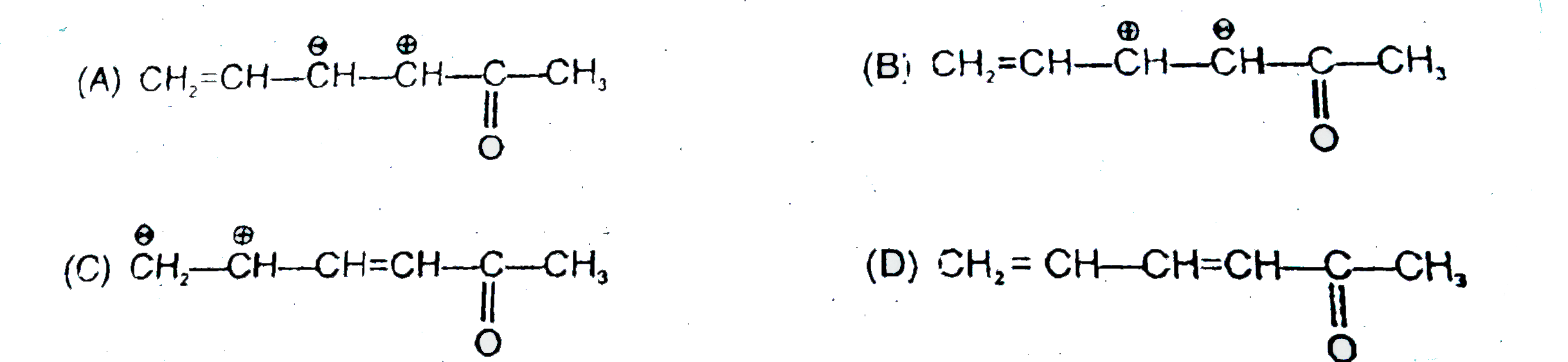 A1,4B2,3C4,1D3,2
A1,4B2,3C4,1D3,2The least and most stable resonating structures respectively are:
 A2,1B2,3C3,1D1,2
A2,1B2,3C3,1D1,2
Which will be the least stable resonating structure ?
View SolutionWhich will be the least stable resonating structure?
View SolutionWhich will be the least stable resonating structure?
View SolutionWhich will be the least stable resonating structure?
View SolutionWhich will be the least stable resonating structure?
View Solution

