Which of the following will react with NaOH to form methanol as a major product ?
A
(CH3)4N+I−
B
(CH3)3S+I−
C
(CH3)3CCl
D
CH3−O−CH3
Text Solution
The correct Answer is:A,B
3∘R−X will afford alkene as major product with NaOH. Due to greater electronegativity of N over S, positive charge on N will make the methyl groups more electron-deficient therefore, (CH3)4N+I− will undergo nucleophilic substitution more readily.
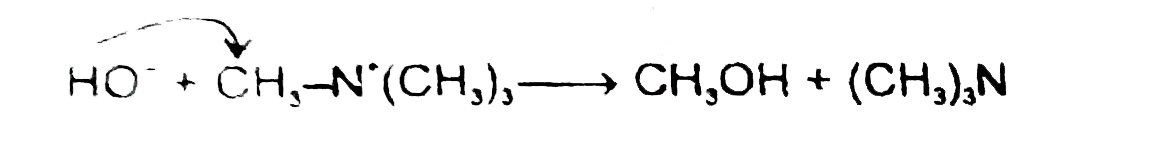
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following will not react with NaOH?
A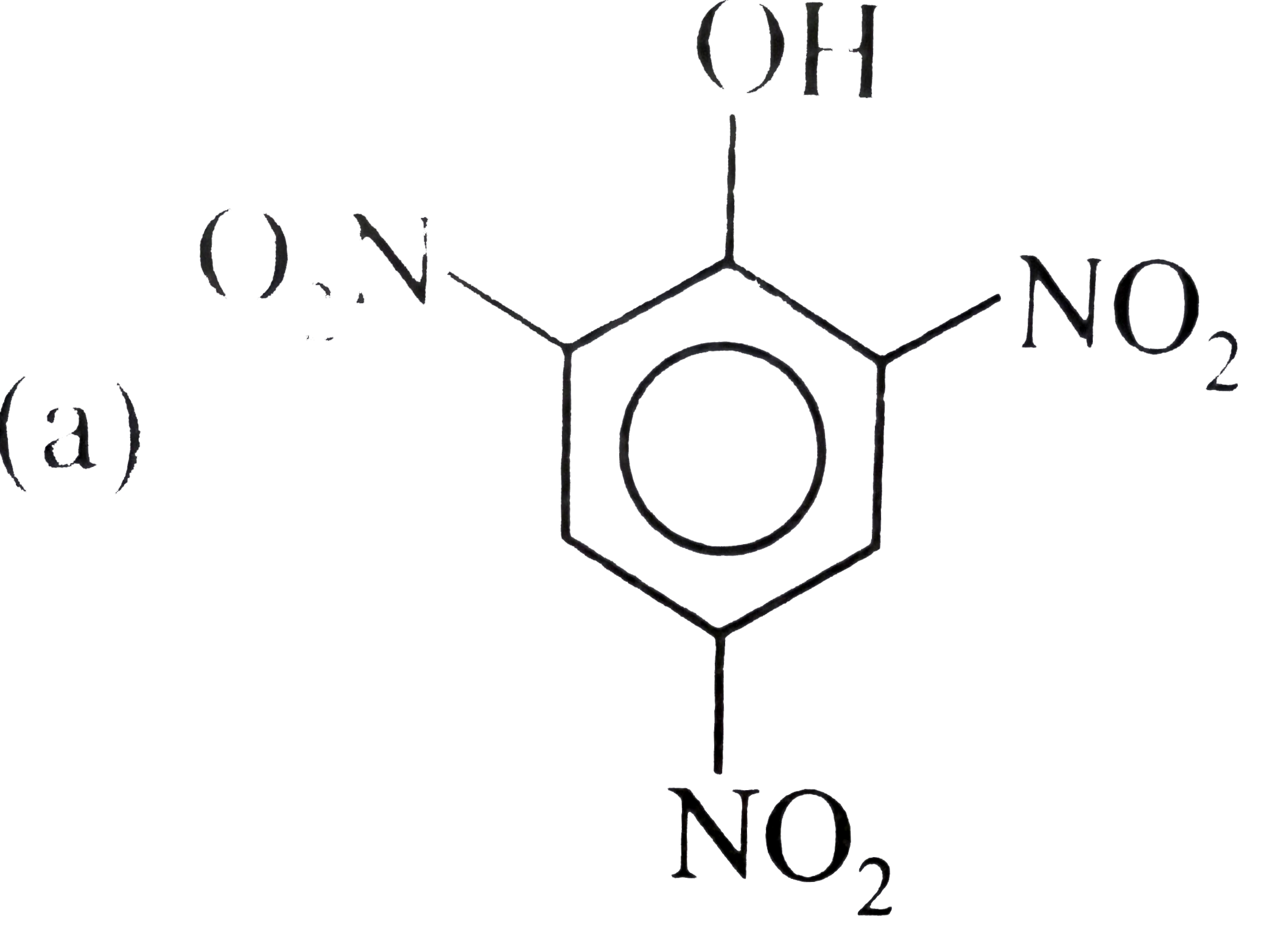 BCH3CONH2CC2H5OHDCH(CN)3
BCH3CONH2CC2H5OHDCH(CN)3Which of the following will not react with NaOH?
A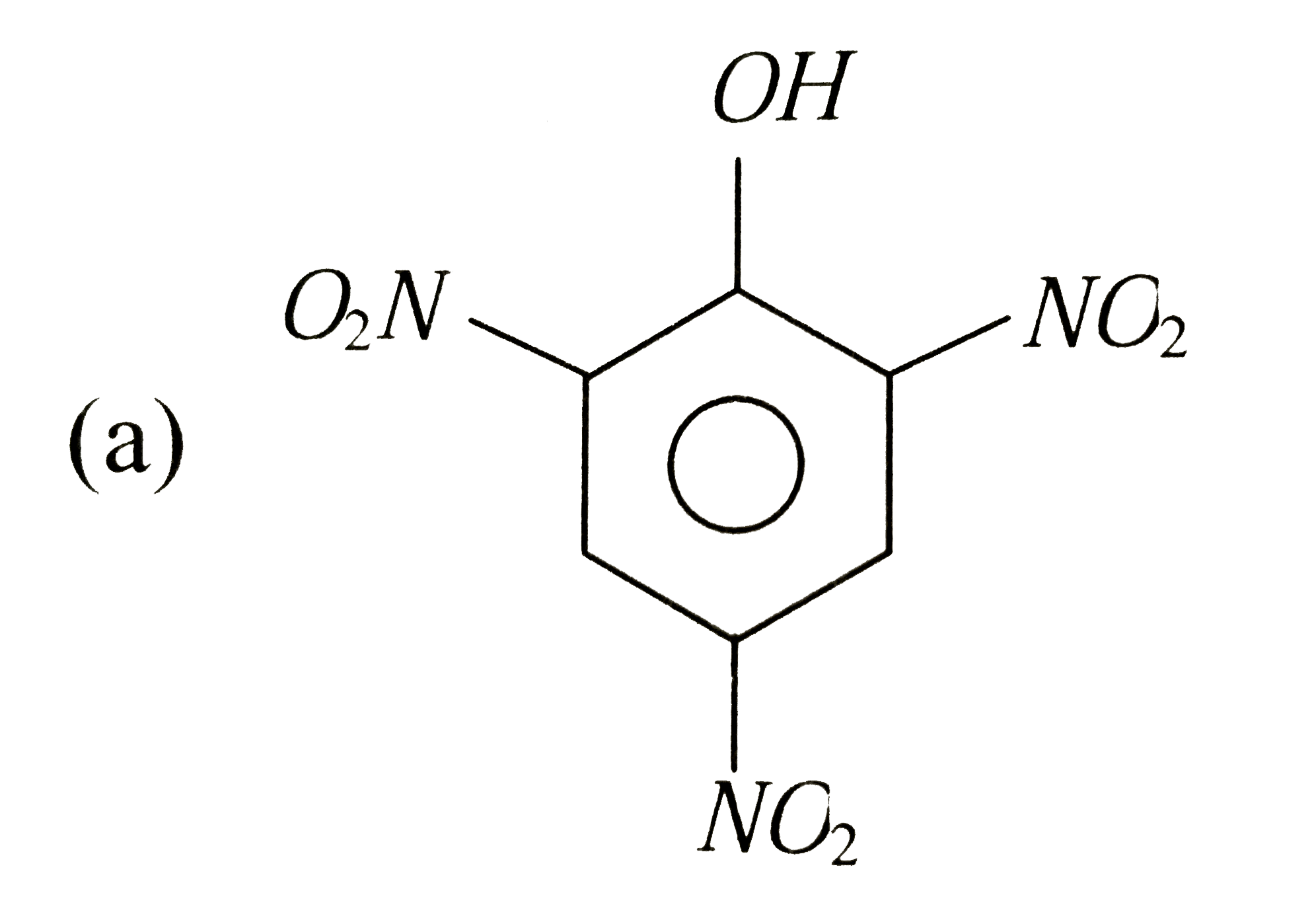 BC2H5OHCCH3CONH2DCH(CN)3
BC2H5OHCCH3CONH2DCH(CN)3Which one of the following reacts with haloalkane of form nitroalkane as a major product?
AKNO2BAgNO2CAgNO3DHNO2
In which of the following Saytzeff product will not be formed as major product?
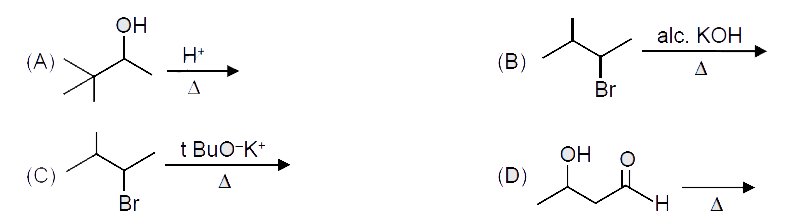
View Solutionwhich of the following product form when dil NaOH react
View SolutionZinc reacts with NaOH to form the following products:
View SolutionThe compound that will react most readily NaOH to form methanol is
View SolutionThe compound that will react most readily with NaOH to from methanol is
View Solution

