Which of the following correctly represents the rate of acid-catalysed hydration of following alkenes.
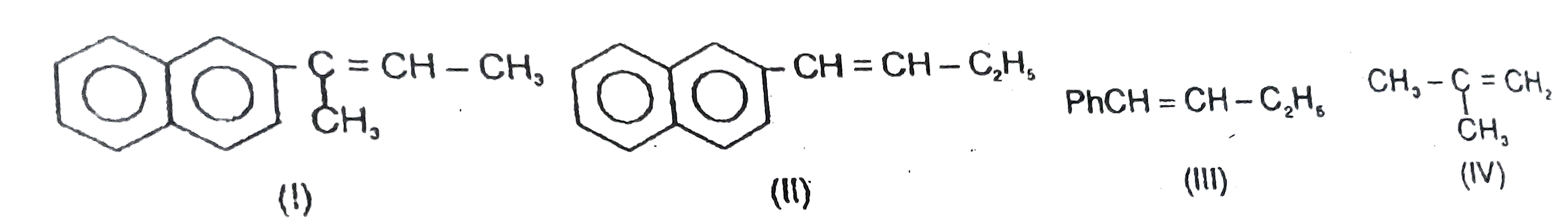
(III)PhCH=CH−C2H6 , (IV)CH3−C∣CH3=CH2
A
IIIgtIgtIIgtIV
B
IVgtIIIgtIgtII
C
IgtIIgtIIIgtIV
D
IVgtIIIgtIIgtI
Text Solution
The correct Answer is:C
rate of according to stability of carbocation formed
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
What is the correct order of acid catalysed hydation of following alkenes ?
(I)CH2=CH2 (II)CH3−CH=CH2 (III)(CH3)2C=CH2 (IV)CH3−CH=CH−CH3AIII gt I gt II gt IVBIII gt IV gt II gt ICII gt IV gt III gt IDI gt II gt III gt IVRates of hydration of the following alkene are:
CH3−OCH=CH2 F−CH=CH=CH2 CH3CH=CH2
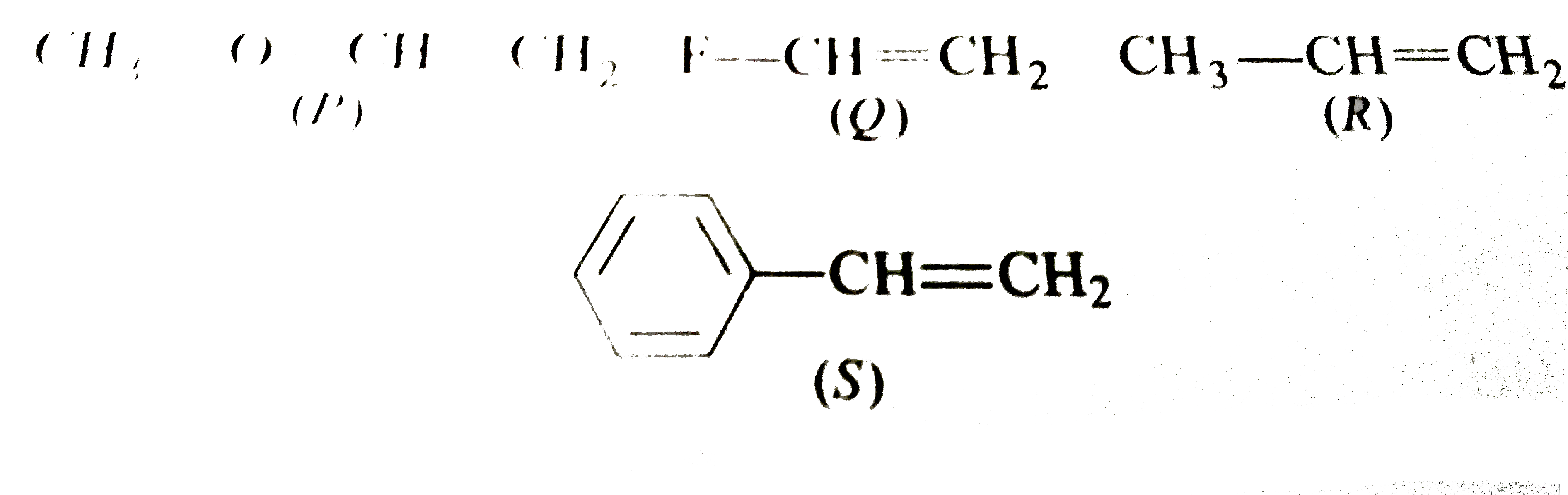 AP>QR>SBS>R>Q>PCP>S>R>QDR>S>P>Q
AP>QR>SBS>R>Q>PCP>S>R>QDR>S>P>QWhich of the following statement are correct for give reaction.
CH3−C∣CH3=CH−CH3+HBrPeroxide−−−−→CH3−Br∣C∣CH3−CH2−CH3 CH3−C∣CH3H−Br∣CH−CH3AMajor product is mixture of two enantiomers.BLess stable carbocation give major productCLess stable free radical give major productDMore stable free radical give major product
Write IUPAC name of the following
(i) , (ii)CH3−CH3∣CH−C≡CH , (iii)CH3−C≡C−CH∣CH3−CH3
, (ii)CH3−CH3∣CH−C≡CH , (iii)CH3−C≡C−CH∣CH3−CH3
View SolutionCompare the stabilites of the following alkenes
H2C=CH3∣C−CH2−CH3
View SolutionArrange the following alkyl halides in decreasing order of the rate or β-elimination reaction alcoholic KOH.
A. CH3−H∣C∣CH3−CH2Br
B. CH3−CH2−Br
C. CH3−CH2−CH2−Br
View SolutionWhat is the major product that will be formed in the following reaction ?
CH3−CH3∣C∣CH3−CH=CH2+HCl→
View SolutionWhich of the following represent the correct order of acidic strength ?
(i)HC≡CH>H2C=CH2>CH3−CH3
(ii) HC≡CH>CH3−CH3>H2C=CH2
(iii) CH3C≡CH>HC≡CH>CH3−C≡C−CH3
(iv)HC≡CH>CH3−C≡CH>CH3−C≡C−CH3
View Solution

