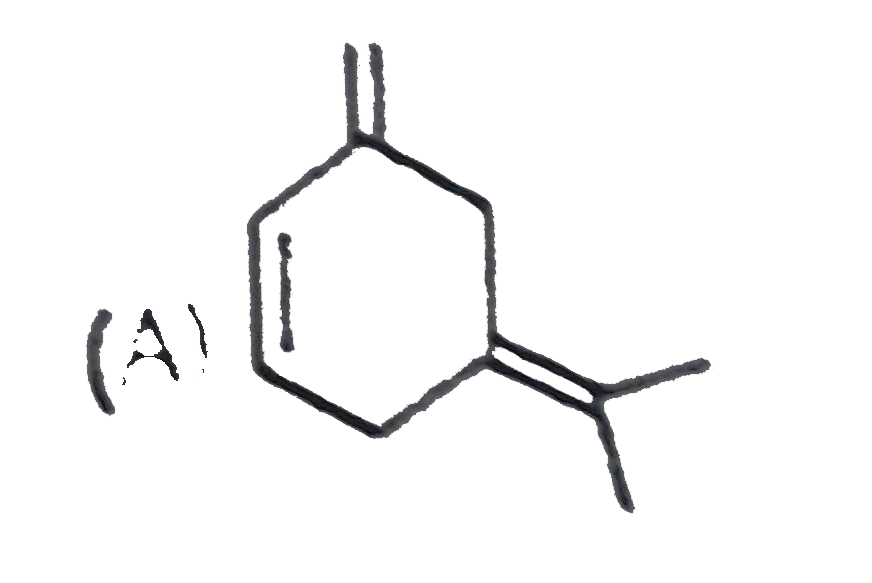
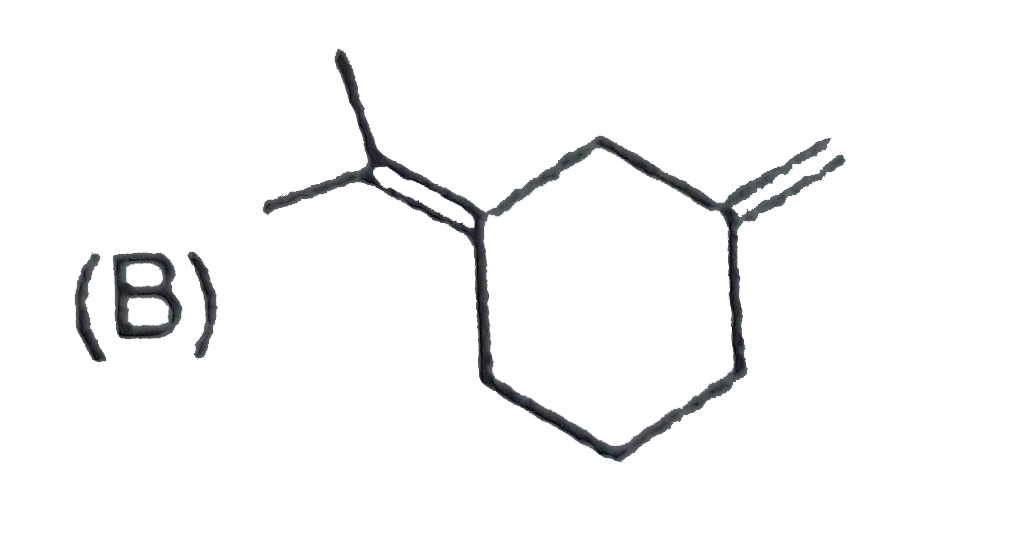
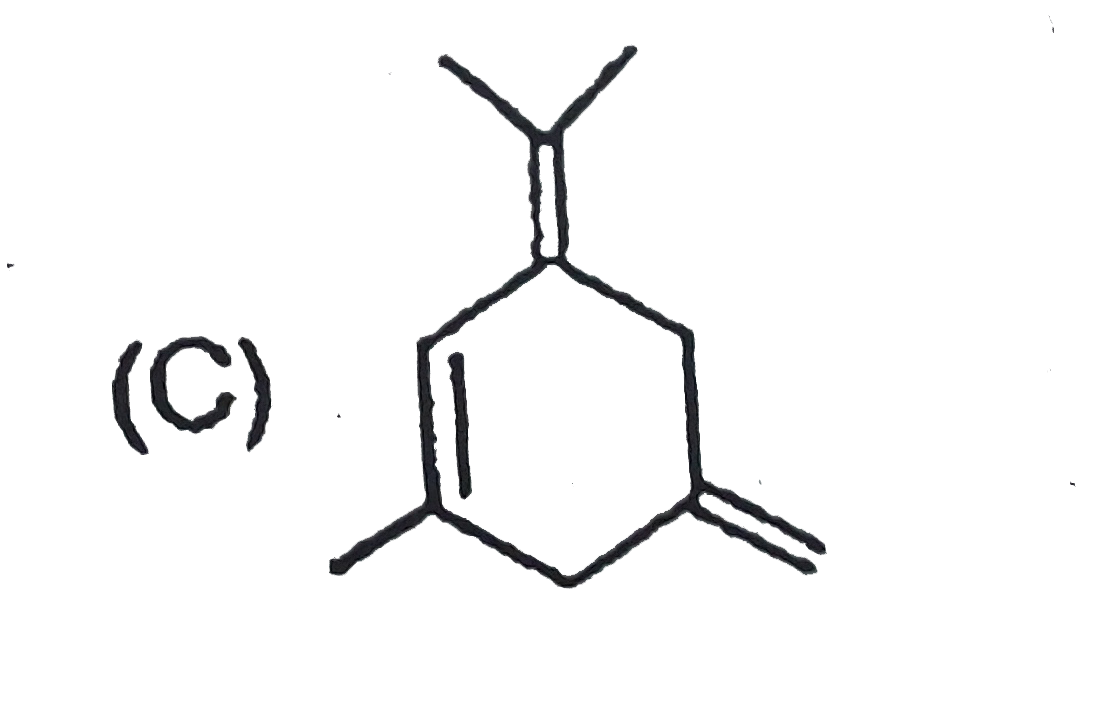
An unsaturated hydrocarbon on complete hydrogenation gives 1-isopropl-3-methylcyclohexane, after ozonolysis it gives one mole of formaldehyde, one mole of acetone and one mole of 2,4-Dixoxohexanedial.The possible structure of the hydrocarbon may be
A
B
C
D
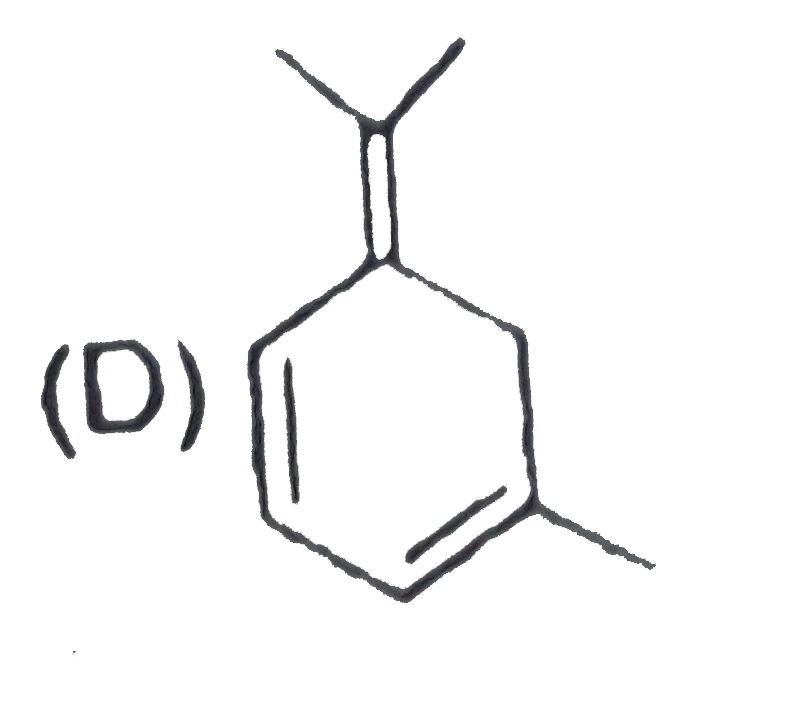
Text Solution
The correct Answer is:A
The following Reaction are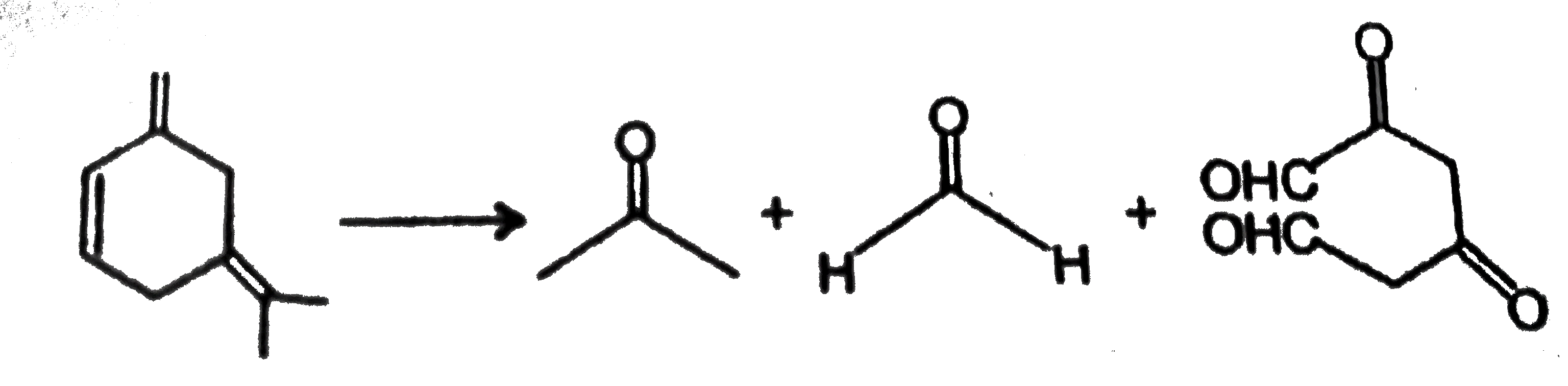
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Farnesence is a compound found in the waxy coating of apples. On hydrogenation it gives 2,6,10-Trimethyl dodecane. On ozonolysis it gives one mole acetone, one mole of formoaldehyde, one mole of 2-Methylpentanedial and one mole of 4-Oxopentanal. The structure proposed for Farnesence may be
A B
B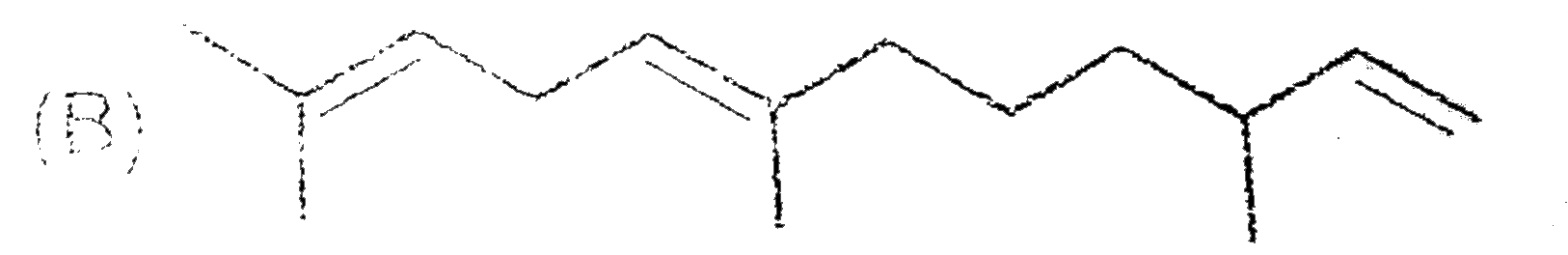 C
C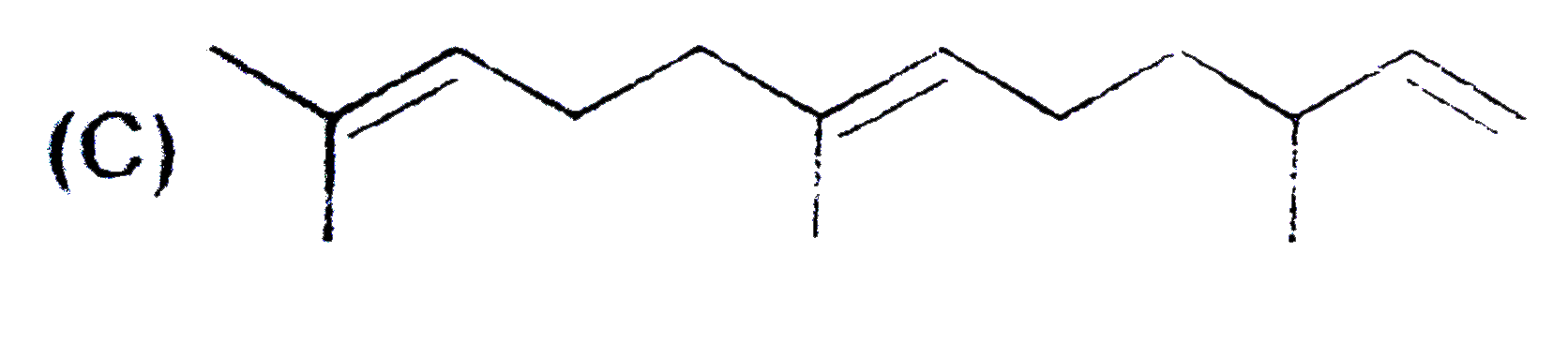 D
D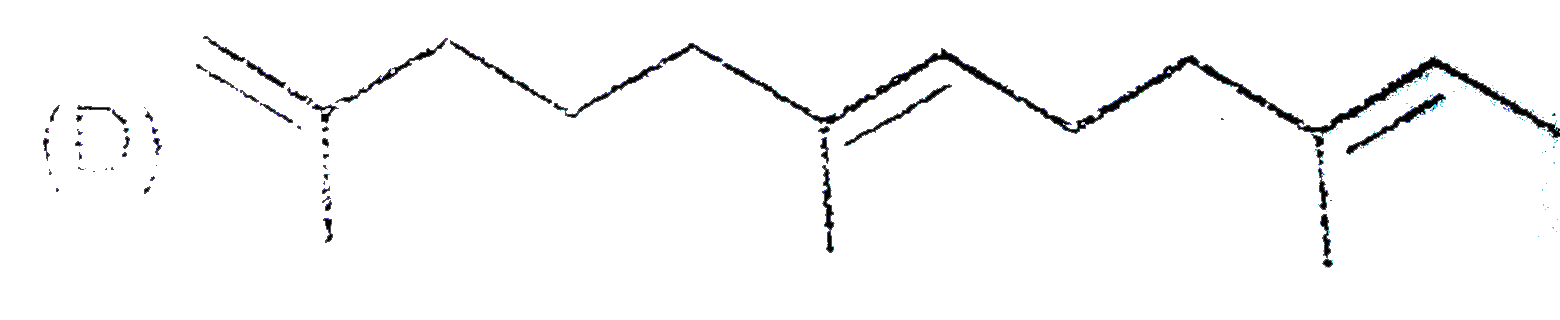
One mole fo X on ozonolysis gave one mole of acetaldehyde and one mole of acetone. The IUPAC name of compound is
A2 methyl 2 buteneB2 methyl 1 buteneC2 ButeneD1-ButeneFarnesene is a compound found in the waxy coating of apples. On hydrogenation it gives 2,6,10-Trimethyl dodecane. On ozonolysis it gives one mole of 2-Methylpentanedial and one mole of 4-Oxopentanal. The structure proposed for Farnesene may be
A B
B C
C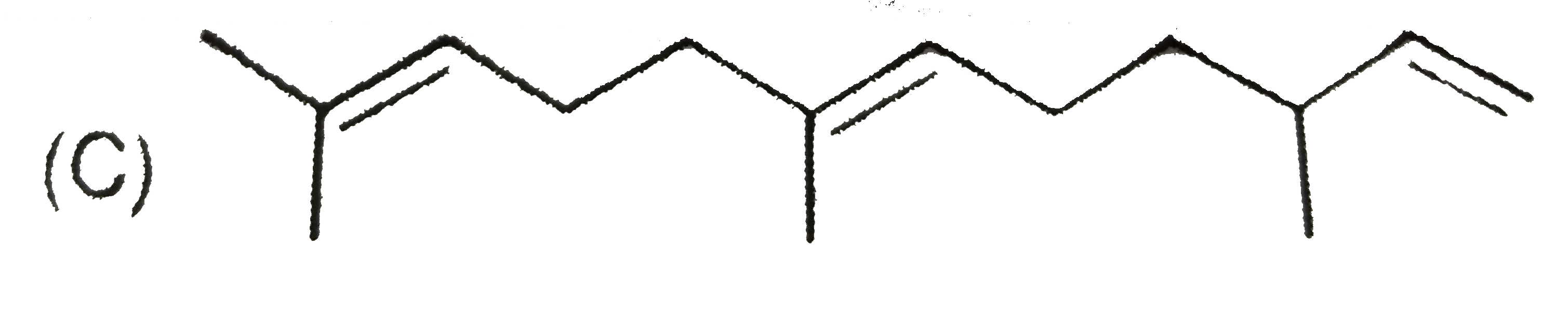 D
D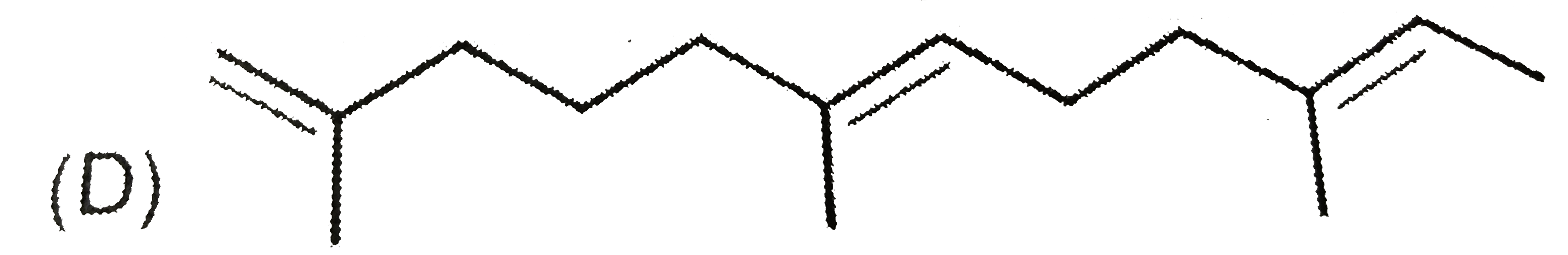
An alkene gives two moles of HCHO, one mole of CO2 and one mole of CH3COCHO on ozonolysis. The structure of alkene is
View SolutionAn alkene gives two moles of HCHO, one mole of CO2 and one mole of CH3COCHO on ozonolysis. The structure of alkene is
View SolutionAn unsaturated hydrocarbon on ozonolysis gives one mole each of methanal, ethanal and 2-ketopropanal. The structure of the hydrocarbon is
View SolutionOne mole of alkene on ozonolysis gives 2 moles of butanone. The alkene is :
View SolutionOne mole of alkene an ozonolysis gives two moles of butanone. The alkene is :
View Solution

