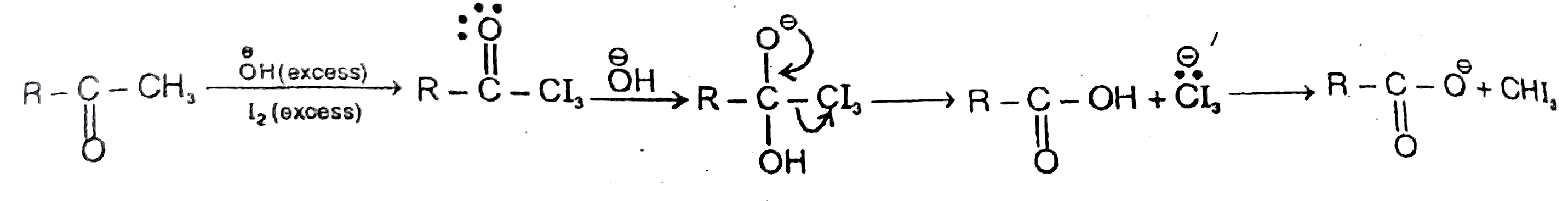
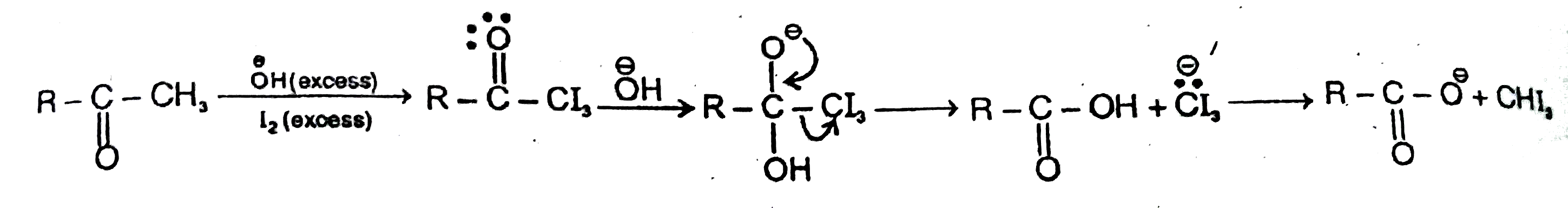
In the presence of excess base and excess halogen a methylketone is converted first into a trihalo substituted ketone and then into a carboxylic acid.After the trihalo substituted ketone is formed hydroxide ion attacks the carboxyl carbon because the trihalo methyl ion is the group more easily expelled from the tetrahedral intermediate.The conversion of a methyl ketone to a carboxylic acid is called a haloform reaction because one of the product is haloform (CHCl3) or CHI3 or CHBr3.
Which of the following compound show haloform reaction and racemisation in OD−/D2O
CH3CH2OH
CH3−C∣∣O−C∣EtH−Ph
The correct Answer is:D
CH3−C∣∣O−CH∣Et−Ph
If H, present on the chiral carbon, undergoes tautomerisation we will get racemic mixture.
Topper's Solved these Questions
Similar Questions
A red solid is insoluble in water.However it becomes soluble if some KI is added to water.Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube.The red solid is :
View SolutionSodium (Na=23) crystallizes in bcc arrangement with the interfacial separation between the atoms at the edge 53.6±. The density of sodium crystal is :
View SolutionIn presence of excess base and exces shalogen a methyl ketone is converted first into a trihalo substituted ketone and then into a carbonylc acid . After the trihalo substituted ketone is formed hydroxide ion attacks the carboxyl carbon because the trihalo methyl ion is the group more easily expelled from the tetrahedral intermediate .The conversion of methyl ketone to a carboxylic acid is called a haloform reaction because one of the product is halofor (CHCl_(3)) or CHI_(3) or CHBr_(3) . Which of the following compoud show haloform reaction and racemisation in OD^(-)//D_(2)O .
View SolutionIn presence of excess base and exces shalogen a methyl ketone is converted first into a trihalo substituted ketone and then into a carbonylc acid . After the trihalo substituted ketone is formed hydroxide ion attacks the carboxyl carbon because the trihalo methyl ion is the group more easily expelled from the tetrahedral intermediate .The conversion of methyl ketone to a carboxylic acid is called a haloform reaction because one of the product is halofor (CHCl_(3)) or CHI_(3) or CHBr_(3) .
View SolutionIn presence of excess base and exces shalogen a methyl ketone is converted first into a trihalo substituted ketone and then into a carbonylc acid . After the trihalo substituted ketone is formed hydroxide ion attacks the carboxyl carbon because the trihalo methyl ion is the group more easily expelled from the tetrahedral intermediate .The conversion of methyl ketone to a carboxylic acid is called a haloform reaction because one of the product is halofor (CHCl_(3)) or CHI_(3) or CHBr_(3) . Product " " is :
View SolutionIn the presence of excess base and excess halogen a methylketone is converted first into a trihalo substituted ketone and then into a carboxylic acid.After the trihalo substituted ketone is formed hydroxide ion attacks the carboxyl carbon because the trihalo methyl ion is the group more easily expelled from the tetrahedral intermediate.The conversion of a methyl ketone to a carboxylic acid is called a haloform reaction because one of the product is haloform (CHCl_3) or CHI_3 or CHBr_3 . Ph-undersetunderset(O)(||)C-undersetunderset(Me)(|)oversetoverset(Et)(|)C-undersetunderset(O)(||)C-CH_3undersetunderset((3)Delta)((2)H^(o+))overset((1)I_2//OH^(o+))to Product is
View SolutionIn the presence of excess base and excess halogen a methylketone is converted first into a trihalo substituted ketone and then into a carboxylic acid.After the trihalo substituted ketone is formed hydroxide ion attacks the carboxyl carbon because the trihalo methyl ion is the group more easily expelled from the tetrahedral intermediate.The conversion of a methyl ketone to a carboxylic acid is called a haloform reaction because one of the product is haloform (CHCl3) or CHI3 or CHBr3.
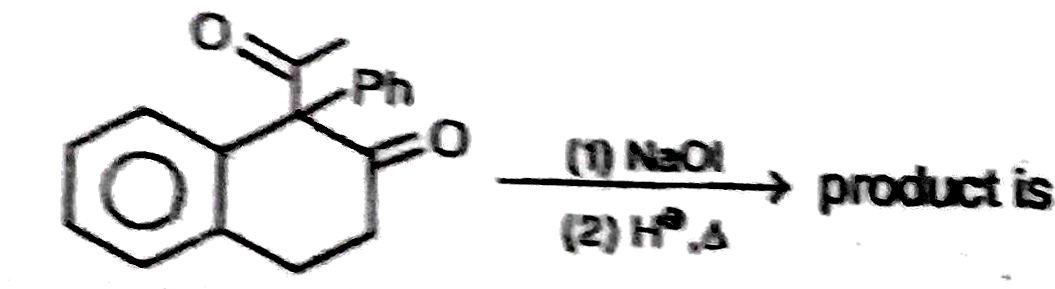
View Solutionऐरोमैटिक कार्बोक्सिलिक अम्लों में इलेक्ट्रॉन स्नेही प्रतिस्थापी केवल ...... परिस्थितियों के अन्तर्गत होता है क्योंकि कार्बोक्सिल समूह एक ..... समूह है |
View SolutionCarboxylic acid can be converted into corresponding ketone by heating their :
View SolutionCarboxylic acid can be converted into corresponding ketone by heating their :
View SolutionAssertion(A): Carboxylic acid do not give the characteristic reaction of carbonyl group as given by aldehyde and ketone.
Reason (R): The carbonyl carbon of carboxylic acid is involved in resonance
View SolutionAldehyde , Ketone And Carboxylic Acid|Gattermann-Koch Reaction
View SolutionAldehydes, Ketones and Carboxylic acids are Carbonyl compounds. Aldehydes differ from Ketones in their oxidation reaction. Illustrate with one example.
View SolutionKetones can NOT be converted into carboxylic acid using____.
View SolutionAldehydes, Ketons And Carboxylic Acids
View Solution

