Identify the elements to complete the table :
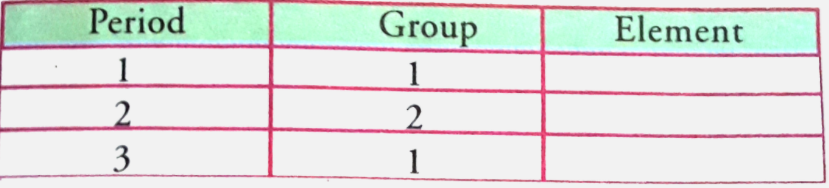
Period (1), Group (1), Element (H), Period (2) Group (2) Element (Be), Period (3), Group (1) Element (Na)
Similar Questions
Complete the given table
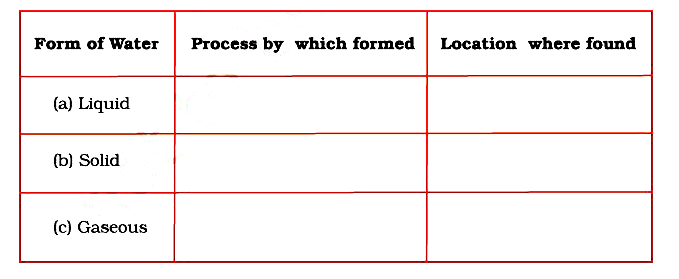
View SolutionComplete the given table

View SolutionUse the periodic table to answer the following questions.
a. Identify an element with five electrons in the outer subshell.
b. Identify an element that would tend to lose two electrons.
c. Identify an element that would tend to gain two electrons.
d. Identify the group having metal, non-metal, liquid as well as gas at the room temperature.
View SolutionIn the modern periodic table, magnesium is surrounded by elements with atomic
numbers 4, 11, 13 and 20. Identify the elements. Which of these have chemical
properties resembling magnesium?
View SolutionComplete the given table.

View SolutionAtomic numbers of a few elements are given below :
10, 20, 7, 14
(a) Identify the elements.
(b) Identify the group number of these elements in the Periodic Table.
(c) Identify the period of these elements in the Periodic Table.
(d) What would be the electronic configuration for each of these elements ?
(e) Determine the valency of these elements.
View SolutionAtomic number of few elements are given below 10, 20, 7, 14
(a) Identify the elements
(b) Identify the group number of these elements in the periodic table
(c) Identify the periods of these elements in the periodic table
(d) What would be the electronic configuration for each of these elements
(e) Determine the valency of these elements.
View SolutionAtomic number of few elements are given below 10, 20, 7, 14
(a) Identify the elements
(b) Identify the group number of these elements in the periodic table
(c) Identify the periods of these elements in the periodic table
(d) What would be the electronic configuration for each of these elements
(e) Determine the valency of these elements.
View SolutionComplete the followig table :

View SolutionUse the periodic table to answer the following questions.
a. Identify an element with five electrons in the outer subshell.
b. Identify an element that would tend to lose two electrons.
c. Identify an element that would tend to gain two electrons.
d. Identify the group having metal, non-metal, liquid as well as gas at the room temperature.
View Solution
