Emprical formula of a hydrocarbon containing 80 % corbon and 20 % hydrogen is
CH
CH2
CH3
CH4
The correct Answer is:C
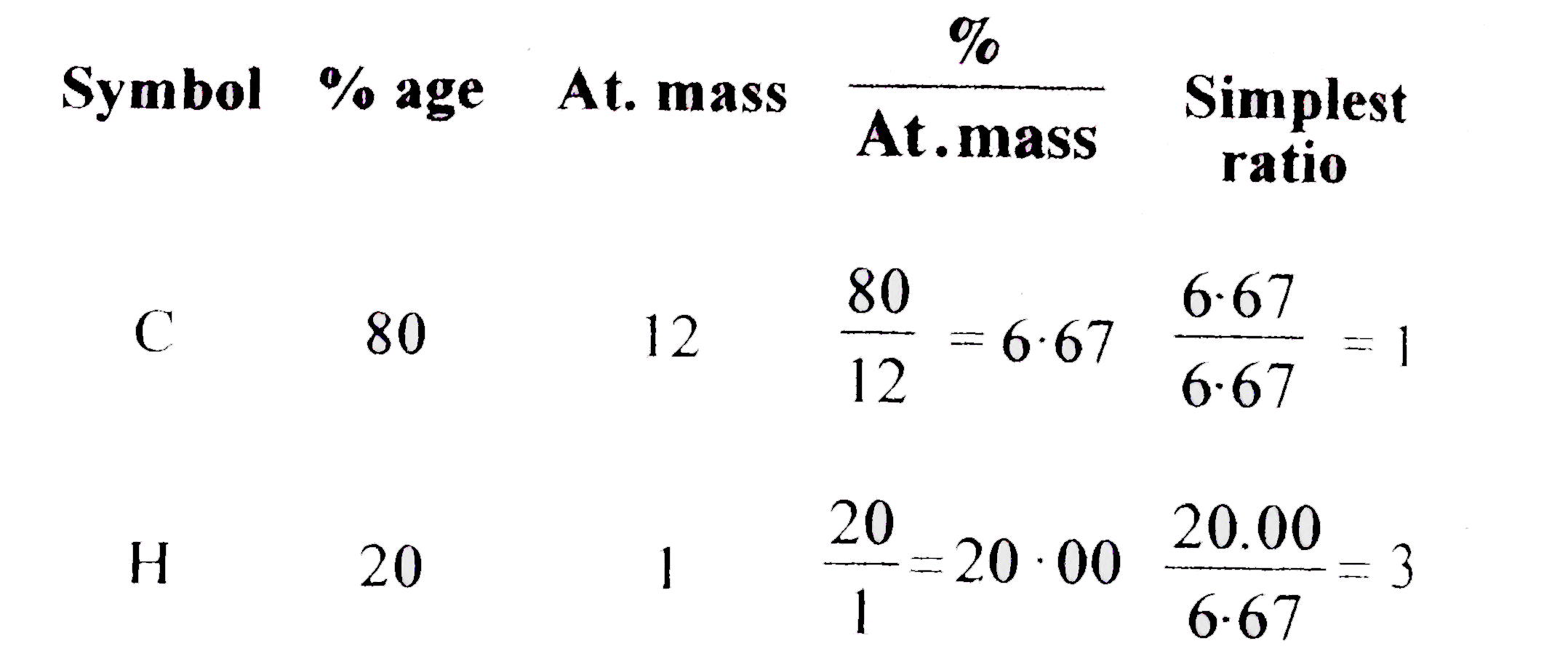
Empirical formula =CH3
Similar Questions
A certain hydrocarbon (A) was found to contain 85.7% carbon and 14.3% hydrogen. This compound consumes 1 molar equivalent of hydrogen to give a saturated hydrocarbon (B). One gram of hydrocarbon (A) just decolourised 38.05gm of a 5% solution (by weight) of Br2 in CCl4. Compound (A) on oxidation with concentrated KMn4 gave compound (C) (molecular formula, C4H8O) and acetic acid. Compound (C) could easily be prepared by the action of acidic aqueous mercuric sulphate on 2-butyne. Determine the molecular formula of (A) and deduce the structures of (A),(B), and (C).
View SolutionA gaseous hydrocarbon contaisn 85.7% carbon and 14.3% hydrogen 1 litre of the hydrocarbon weights 1.26g at NTP. Determine the molecular formula of the hydrocarbon.
View Solution
Knowledge Check
Empricial formula of a hydrocarbon containing 80 % carbon and 20% hydrogen is
ACHBCH2CCH3DCH4Empricial formula of a hydrocarbon containing 80 % carbon and 20% hydrogen is
ACHBCH2CCH3DCH4A hydrocabron coantaing 80% carbon, then the hydrocarbon is :-
ACH4BC2H4−CC2H2DC3H9
Ammonia contains 82.32% of nitrogen and 17.65 % of hydrogen . Water contains 88.90% of oxygen and 11.10% of hydrogen . Nitrogen trioxide contains 63.15% of oxygen and 36.85% of nitrogen. Show that these data illustrate the law of reciprocal proportions.
View SolutionThe empirical formula of a compound containing 50% of an element A (atomic mass = 10) and 50% of another element B (atomic mass = 20) is
View SolutionAn organic compound containing carbon hydrogen and oxygen contains 52 .2 % carbon and 13.04 % hydrogen .Vapour density of the compound is 23 .Its molecular formula will be
View SolutionA compound containing 80% C and 20% H is likely to be
View SolutionA compound containing 80% C and 20% H is likely to be:
View Solution
