The molecular shapes of diborane is shown below :
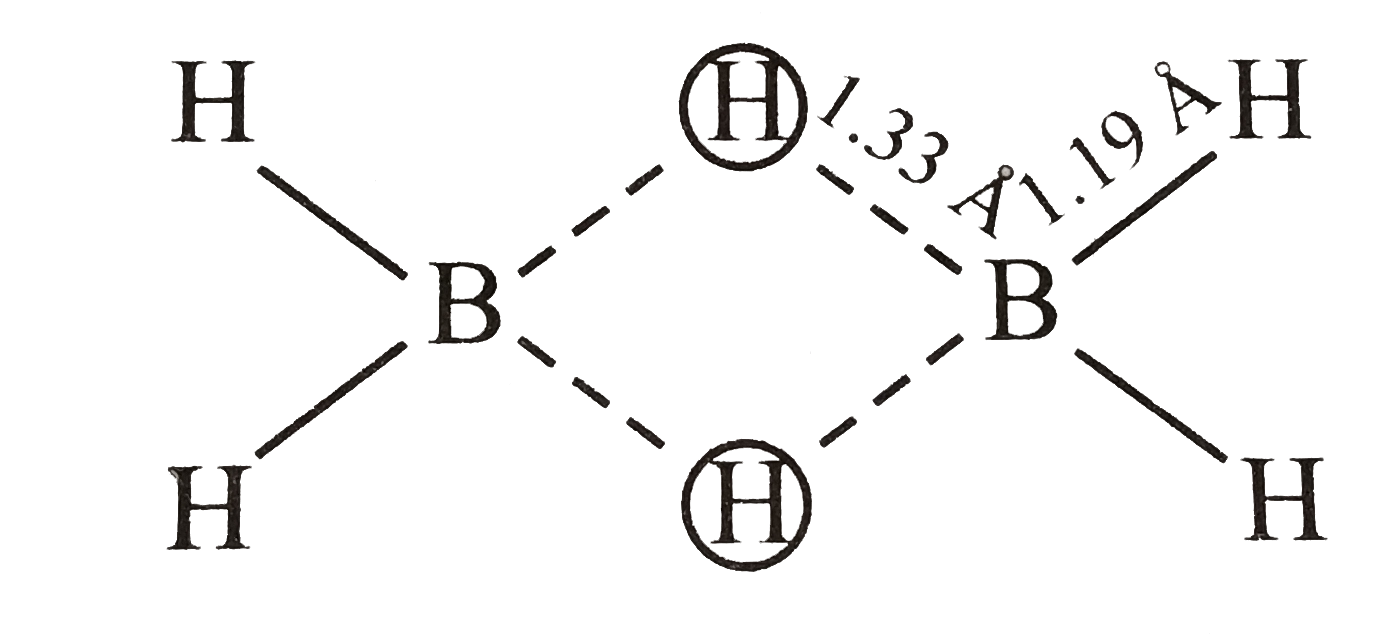 .
.
Consider the following statements for diborane :
(i) Boron is approximately sp3 hybridised.
(ii) B−H−B angle is 180∘
(iii) There are two terminal B−H bonds for each boron atom.
(iv) There are only 12 bonding electrons available of These statements :
1, 3 and 4 are correct
1, 2 and 3 are correct
2, 3 and 4 are correct
1, 2 and 4 are correct.
The correct Answer is:A
Step by step video, text & image solution for The molecular shapes of diborane is shown below : . Consider the following statements for diborane : (i) Boron is approximately sp^(3) hybridised. (ii) B-H-B angle is 180^@ (iii) There are two terminal B-H bonds for each boron atom. (iv) There are only 12 bonding electrons available of These statements : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Consider the following statements, (i) Diborane contains two centre two electron bond. (ii) In diborane, the boron has sp^(3) hybridised. (iii)Diborane has two terminal B-H bond and four B-H-B bonds. Which of the above statements (s) is/are correct.
View SolutionDiborane has ……………….. B-H bonds.
View SolutionDiborane is simplest and most familiar hydride of boton. Its chemical formula is B2H6. Comparing with ethane, diborane is regarded as electron deficient molecule. In the excited state boron of diborane undergoes sp3 hybridisation. Bonding in doborane is described as tricentric two electron bonding.
Structure of −BH2 group is
View SolutionAssertion : In diborane, each B atom is sp2 hybridised.
Reason : In diborane, the terminal 2-centre-2-electron B-H bonds are called banana bonds.
View SolutionConsider the following statement for diborane: Boron is approximately sp^3 hybridized B-H-B angle is 180^@ There are two termine B-H bonds for each boron atom There are only 12 bonding electrons available of these statements :
View SolutionThe molecular shapes of diborane is shown below :
 .
.
Consider the following statements for diborane :
(i) Boron is approximately sp3 hybridised.
(ii) B−H−B angle is 180∘
(iii) There are two terminal B−H bonds for each boron atom.
(iv) There are only 12 bonding electrons available of These statements :
View SolutionThe molecular shapes of diborane is shown below : . Consider the following statements for diborane : (i) Boron is approximately sp^(3) hybridised. (ii) B-H-B angle is 180^@ (iii) There are two terminal B-H bonds for each boron atom. (iv) There are only 12 bonding electrons available of These statements :
View SolutionIf diborane each boron has three electrons bonds around each B atom and form
View Solution

