The molecular shapes of diborane is shown below :
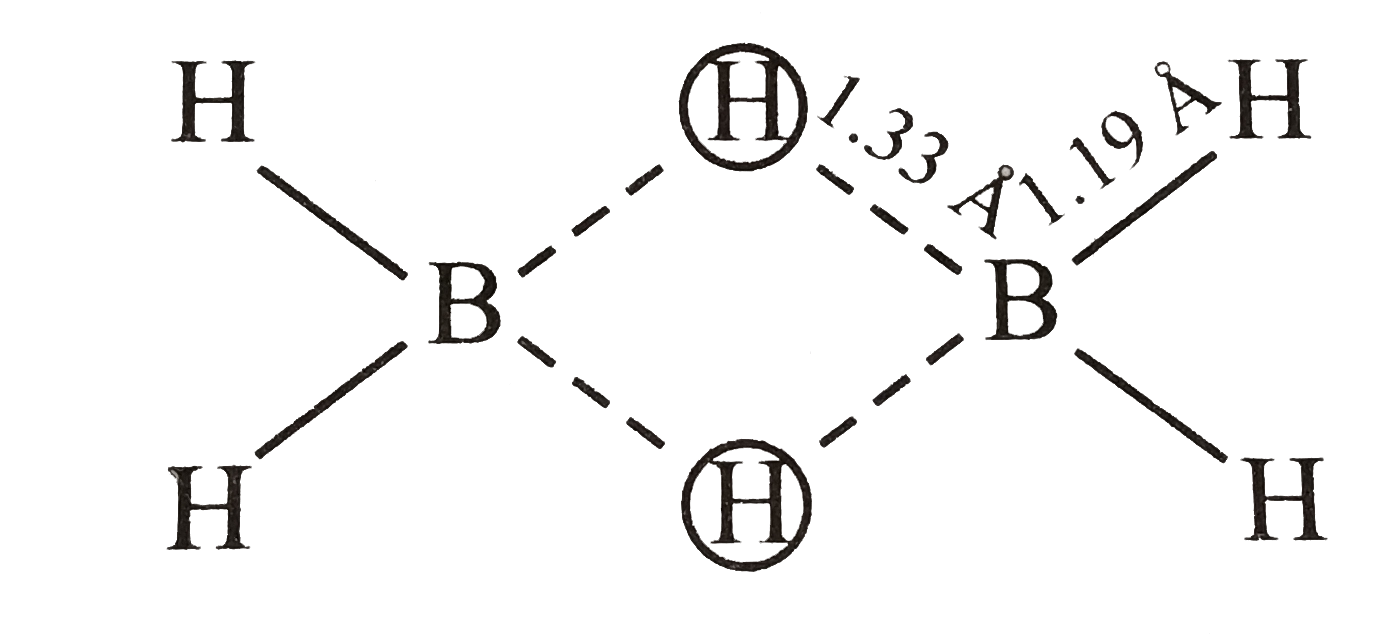 .
.
Select correct statement about B2H6 :
A
Bridging group are electron deficient with 12 valence electrons
B
It has 2c-2e B-H bonds
C
It has 3c-2e B-H-B bonds
D
All the above and correct statements.
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for The molecular shapes of diborane is shown below : . Select correct statement about B_(2)H_(6) : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The correct statement about B2H6 is:
ATerminal B-H bonds have less p-character when compared to briging bondsBThe two B-H-B bonds are not of same lengthCAll B-H-B angles are of 120∘DIts fragment ,BH3, behaves as a Lewis baseSelect the incorrect statement for B2H6
AIt contains B-B ionic bondBEach boron is sp3 hybridisedCit has two types of hydrogen bondsDAll of theseSelect the incorrect statement for B2H6
AIt contains B- B ionic bondsBEach boron is sp3 hybridisedCIt has two types of hydrogen bondsDAll of these
