Which of the following reaction are possible ?
(1) ArN+2+CuBr→Ar−Br
(2)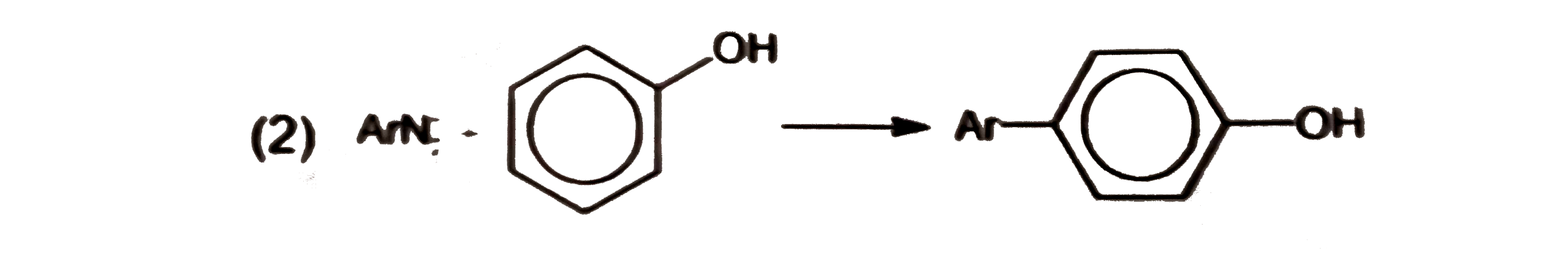
(3) ArN+2+H3PO2H2O−−→ArH
(4) ArN+2+I−→ArI
Select the answer using codes given below:
1, 2 and 4
1, 3 and 4
1, 2 and 3
2, 3 and 4
The correct Answer is:B
Step by step video, text & image solution for Which of the following reaction are possible ? (1) ArN_(2)^(+) + CuBr rarr Ar -Br (2) (3) ArN_(2)^(+) + H_(3)PO_(2) overset(H_(2)O)rarr ArH (4) ArN_(2)^(+) + I^(-) rarr ArI Select the answer using codes given below: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Equivalent weight of KmNO4 when it is convert into MnSO4 is Where M = molar mass of KMnO4.
View SolutionWhen a transition of electron in He+ takes place from n2 to n1 then wave number in terms of Rydberg constant R will be
(Given n1+n2=4,n2−n1=2)
View SolutionWhich of the following reaction are possible ? (1) ArN_(2)^(+) + CuBr rarr Ar -Br (2) (3) ArN_(2)^(+) + H_(3)PO_(2) overset(H_(2)O)rarr ArH (4) ArN_(2)^(+) + I^(-) rarr ArI Select the answer using codes given below:
View SolutionRank the transition states that occur during the following reaction steps in order of increasing stability (least → stable)
1. H3C−+OH2→CH+3+H2O
2. (CH3)3C−+OH2→(CH3)3C++H2O
3. (CH3)2CH−+OH2→(CH3)2CH++H2O
View SolutionHow many of the following reaction would produce phosphine gas ?
(a) PH4Cl+NaOH→ (b)P4+NaOHΔ−−→
( c) Ca3P2+H2O→ (d)H3PO3Δ−−→
(e ) PCl5+H2SO4→ (f)H3PO2Δ−−→
(g) P2Cl4+H2O→ (h)AlP+H2OΔ−−→
(i) P4O10+H2O→
View SolutionWhich of the following reactions will yield propan-2-ol ? Select the right answer from (a), (b), (c) and (d) I. CH_(2)=CH-CH_(3)+H_(2)O overset(H^(+))(rarr) II. CH_(3)-CHO overset(CH_(3)MgI//H_(2)O)(rarr) III. CH_(2)O underset(H_(2)O)overset(C_(2)H_(5)MgI)(rarr) IV. CH_(2)=CH-CH_(3) overset("Neutral "KMnO_(4))(rarr)
View SolutionWhich of the following reactions will occur ? (1) ArN_(H)^(+) Cl^(-) + CuBr rarr ArBr + CuCl + N_(2) (2) (3) ArN_(H)^(+) + Cl + [H] overset(H_(3)PO_(2) // H_(2)O)rarr ArH + N_(2) + Cl^(-) (4) Ar N_(2)^(+) Cl_(-) + I^(-) rarr Ar I + N_(2) + Cl^(-) Select the correct answer using the codes given below
View SolutionThree reactions involving H2PO−4 are given below
I.H3PO4+H2O→H3O++H2PO−4
II.H2PO−4+H2O→HPO2−4+H3O+
III.H2PO−4+OH−→H3PO4+O2+
In which of the above does H2PO−4 act as an acid?
View SolutionThree reactions involving H2PO−4 are given below
I.H3PO4+H2O→H3O++H2PO−4
II.H2PO−4+H2O→HPO2−4+H3O+
III.H2PO−4+OH−→H3PO4+O2+
In which of the above does H2PO−4 act as an acid?
View Solutionअभिक्रिया ArN_(2)^(+)X-overset(Cu//HBr)toAr-Br+N_(2)+CuX कहलाती हैं-
View SolutionWhat is Y in the following reaction sequence
C6H5N2ClH3PO2/H2O−−−−−−−→XCO.HCl−−−−−−−−→Anhy.AlCl3
View SolutionIn the reaction : HC -= CH overset(NaNH_(2))rarr X overset(CH_(3)I)rarr Y underset(HgSO_(4))overset(H_(2)O, H_(2)SO_(4))rarr Z, Z is :
View SolutionThree reactions involving H_(2)PO_(4)^(-) are given below : (i) H_(3)PO_(4)+H_(2)Orarr H_(3)O^(+)+H_(2)PO_(4)^(-) (ii) H_(2)PO_(4)^(-)+H_(2)OrarrHPO_(4)^(2-)+H_(3)O^(+) (iii) H_(2)PO_(4)^(-)+OH^(-)rarr H_(3)PO_(4)+O^(2-) In which of the above does H_(2)PO_(4)^(-) act as an acid?
View SolutionThe reaction ArN_(2)^(+)Cl^(-) overset(Cu//HCl)(rarr)ArCl+N_(2)+CuCl is named as …………….
View SolutionArN2Cldil.H2SO4−−−−−−→X+N2+HCl
It Regarding X, correct statement is
View SolutionThe reaction Aroverset(ɵ)(N)_(2)overset(o+)(C )l+Cu + HCl rarr ArCl + Na_(2) + CuCl is known as
View Solution
