The equilibrium
SO2CI2(g)⇔SO2(g)+CI2(g)
is attained at 25∘C in a closed container and inert gas helium is introduced . Which of the following statements is/are correct ?
concentration of SO2,CI2 and SO2CI2 change
More chlorine is formed
concentration of SO2 is reduced
More SO2CI2 is formed
On adding inert gas, concentrations do not change (because volume is constant). Equilibrium is not disturbed.
Topper's Solved these Questions
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - COMPREHENSION
3 Videos
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATRIX -MATCH TYPE QUESTION
1 Video
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - SOLVED PROBLEMS (SUBJECTIVE)
16 Videos
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - NUMERICAL BASED QUESTIONS
2 Videos
- CHEMICAL KINETICS AND RADIOACTIVITYBOOK - FIITJEECHAPTER - CHEMICAL KINETICS AND RADIOACTIVITYEXERCISE - Exercise
9 Videos
Similar Questions
A reaction is spontaneous if free energy change ΔG , for the reaction is negative . The question arises, how we can explain , the spontaneous of reversible reaction taking the example of dissociation equilibrium N2O4⇔2NO2(g) the variation of free energy with the fraction of N2O4 dissociated under standard conditions is shown in the figure below.
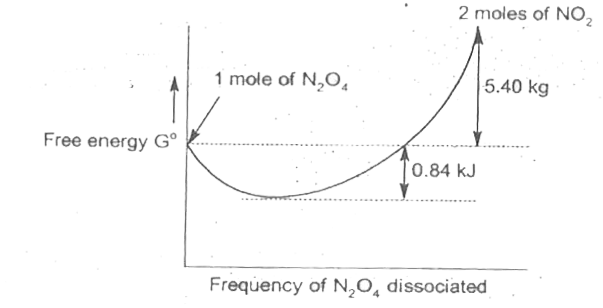
The standard free energy change for the conversion of 1 mole of N2O4 into 2 moles of NO2 is
View SolutionMatch the following column ,
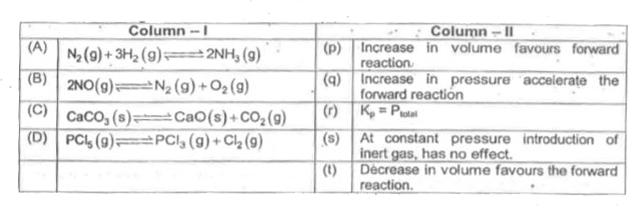
View SolutionConsider the equilibrium A(g)⇔2B(g)+3C(g)at25∘C. When A is loaded into a cylinder at 10.0 atm and the system is allowed to come an equilibrium the , final pressure is found to be 15.76 atm, what is standard Gibbs eneergy change for the reaction ?
View SolutionCalculate the ratio of Z in bcc of fcc?
View SolutionThe rate of formation of NO(g) in the reaction 2NOBr(h)→2NO(g)+Br2(g) was reported as 1.6×10−4mol−1sec−1. What is the rate of reaction and rate of consumption of NOBr?
View SolutionThe equilibrium SO_(2)Cl_(2)(g) hArr SO_(2)(g)+Cl_(2)(g) is attained at 25^(@)C in a closed container and an inert gas, helium, is introduced. Which of the following statement is // are correct?
View SolutionThe equilibrium SO2Cl2(g)⇔SO2(g)+Cl2(g) is attained at 25∘C in a closed container and an inert gas, helium is introduced. Which of the following statements are correct?
View SolutionThe equilibrium , SO2Cl2(g)⇔SO2(g)+Cl2(g) is attained at 25∘C in a closed container and an inert gas , helium , is introduced. Which of the following statement is are correct ?
View SolutionThe equilibrium
SO2Cl2(g)⇔SO2(g)+Cl2(g)
is attained at 25∘C in a closed container and inert gas helium is introduced. Which of the following statement /s is / are correct ?
View SolutionThe equilibrium SO2Cl2(g)⇔SO2(g)+Cl2(g) is attained at 25∘C in a closed container and an inert gas, helium, is introduced. Which of the following statement is/are correct?
View SolutionThe equilibrium
SO2CI2(g)⇔SO2(g)+CI2(g)
is attained at 25∘C in a closed container and inert gas helium is introduced. Which of the following statement (s) is/are correct ?
(1).concentrations of SO2,CI2 and SO2CI2 change
(2). More chlorine is formed
(3).Concentration of SO2 is reduced
(4).More SO2CI2 is formed
View SolutionThe equilibrium , PCI5(g)⇔PCI3(g)+CI2(g) is attatined at 25∘C in a closed container and an inert gas He is introduced . Which of the following statements are correct ?
View SolutionThe equilibrium SO2Cl2(g)⇔SO2(g)+Cl2(g) is attained at 25∘C in a closed container and an inert gas helium is introduced. Which of the following statements is correct ?
View SolutionThe equilibrium of the given reaction
SO2Cl2(g)⇔SO2(g)+Cl2(g)
in attained at 25∘C in a closed container and an inert gas, helium is introduced. Which of the following statement is correct?
View Solution25°C पर बंद पात्र में साम्य SO2Cl2(g)<⇒SO2(g)+Cl2(g) स्थापित होता है और उसमें अक्रिय गैस हीलियम को प्रवेश कराया जाता है तो निम्न में से कौनसा कथन सत्य है
View Solution

