What volume of 3 molar HNO3 is needed to oxidise 8g of Fe3+, HNO3 gets converted to NO ?
8ml
16ml
32ml
64ml
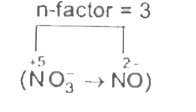
Meq. of HNO3= Meq. of Fe2+
or 3×3×V=856×1000
V=15.87 ml
Similar Questions
Knowledge Check
What volume of 3 molar HNO3 is needed to oxidise 8g of Fe3+, HNO3 gets converted to NO ?
A8mlB16mlC32mlD64mlWhat volume of3MHNO3 is needed to oxidize 8 gFe(2+)→Fe(3+),HNO3 of to gets converted to NO ?
A8 MlB16 mLC32 mLD64 mLHNO3 acts as
AAcidBOxidising agentCReducing agentDBoth (a) and (b)
pH of 10−8MHNO3 is (nearly)
View SolutionHNO3 is manufactured by:
View SolutionOne mole of As2S3 is oxidized by HNO3 to H3AsO4 and H2SO4.HNO3 is converted into NO. The moles of HNO3required are :
View SolutionWhat weight of HNO3 is needed to convert 62gm of P4 in H3PO4 in the region ?
P4+HNO3→H3PO4+NO2+H2O
View SolutionThe equivalent weight of a species if acts as oxidant or reductant should be derived by :
Eq. weight of oxidant or reductant
=Mol. wt. of oxidant or reductantNumber of electrons lost or gained by onemoleculae of oxidant or reductant
During chemical reactions, equal equivalents of one species react with same number of equivalents of other species giving same number of equivalent of products. However this is not true for reactants if they react in terms of moles. Also Molarity can be converted to normality by multiplying the molarity with valence factor or 'n' factor.
One mole of As2S3 is oxidised by HNO3 to H3AsO4 and H2SO4.HNO3 is converted into NO. The moles of HNO3 required are:
View Solution
