Which equiations (s) might be used to illustrate the action of nitric acid as an oxidant ?
A
P4O10+4HNO3→4HPO3+2N2O5
B
6Fe2++8HNO3→6Fe3++2NO+4H2O+6NO−3
C
CO2−3+2HNO3→CO2+H2O+2NO−3
D
Cu+4HNO3→Cu2++2NO−3+2H2O+2NO2
Text Solution
The correct Answer is:B, D
|
Similar Questions
Knowledge Check
A nuclei acid probe might be used to-
AInsert genes into a host cellBMake DNA for gene cloningCSplice pieces of DNADFind a nucleotide sequenceGroup 2 nitrates are prepared in solution by the action of nitric acid on
AoxidesBhydroxidesCcarbonatesDall of theseWhich method (s) can be used to determine the concentration of HNO3 in an aqueous solution of nitric acid ?
(P) Titration with a standard base
(Q) Titration with a standard oxidizing agent
(R) Precipitation with Ag+AP onlyBR onlyCP and Q onlyDP,Q and R
Describe the action of dil. Nitric acid reagents on glucose.
View SolutionState how you would obtain
(i) hydrogen
(ii) nitric oxide
(iii) nascent chlorine - from nitric acid . State the concentration of nitric acid used in each case.
View SolutionIdentify the gas in the following :
Gas produced by the action of dilute nitric acid on zinc .
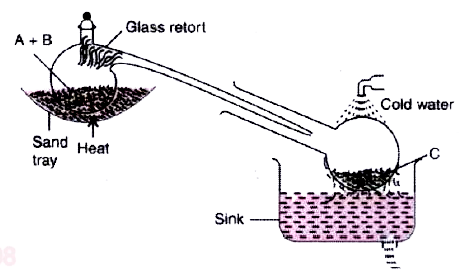
View SolutionThe figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
Write an equation to show how nitric acid undergoes decomposition.
View SolutionWhich one of the following nitrogen oxides is an anhydride of nitric acid?
View Solution

