Excited atoms emits radiations consisting of only certain discrete frequencies or wavelengths. In spectroscopy it is often more convenient to use frequencies or wave numbers than are proportional to energy and spectroscopy involves transitions between energy levels. The line spectrum shown by and mono electronic excited atom (a finger pring of an atom) can be given as
1λ=¯v=RAZ2[1n21−1n22]
When Z is atomic number of mono electronic atom and n1,n2 are integers and if n2>n1, then emission spectrum is noticed and if n2<n1, then absorptioni spectrum is noticed. Every line in spectrum can be represented as differences of two terms RAZ2n21 and RAZ2n22
The given diagrma indicates the energ levels of certain atom. Where an electron moves from 2E.
level to E level, a photon of wavelength of photon emitted during its transition from 4E3 level to E level.
λ3
3λ4
4λ4
3λ
The correct Answer is:D
Topper's Solved these Questions
- ATOMIC STRUCTUREBook:FIITJEEChapter:ATOMIC STRUCTUREExercise:COMPEREHENSION-III
Explore 5 Videos
- ATOMIC STRUCTUREBook:FIITJEEChapter:ATOMIC STRUCTUREExercise:COMPEREHENSION-IV
Explore 3 Videos
- ATOMIC STRUCTUREBook:FIITJEEChapter:ATOMIC STRUCTUREExercise:COMPEREHENSION-I
Explore 3 Videos
- ALKYL AND ARYL HALIDESBook:FIITJEEChapter:ALKYL AND ARYL HALIDESExercise:NUMERICAL BASED QUESTIONS
Explore 1 Video
- CARBOHYDRATES, AMINO ACIDS, POLYMERS AND PRACTICAL ORGANIC CHEMISTRYBook:FIITJEEChapter:CARBOHYDRATES, AMINO ACIDS, POLYMERS AND PRACTICAL ORGANIC CHEMISTRYExercise:EXERCISE
Explore 4 Videos
Similar Questions
When a particle as an electron exist in a box, three quantum numbers are necessary to describe the spatial distributioni fo electrons in atoms. The describe an electron in an atom completely, a fourth quantum number, ms called the spin quantum number must be specified. This is because every electron has magnetic moment associated with it which is quantized in one of two possible orientations. Electrons having the same spin strongly repel each other and tend to occupy different region of space. This result of a fundamental law of nature is known as Pauli exclusion principle.
The quantum numbers +12 and −12 for the electron spin represent
View SolutionWhich writing the following electron configuration of Fe some rule have been violated :
(I) Aufbau principal
(II) Hund's rule
Pauil's exclusion principle
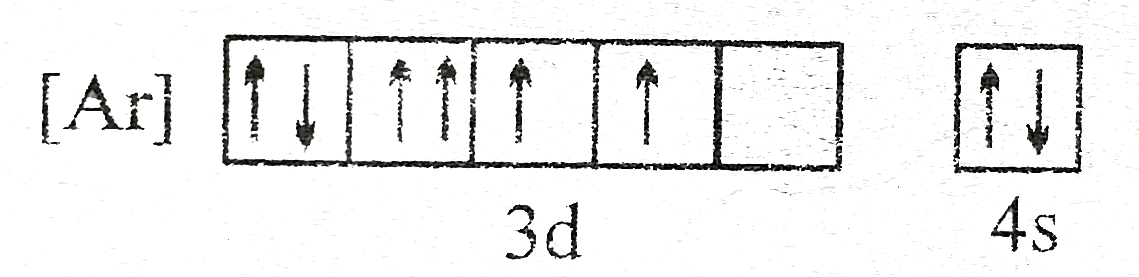
View SolutionA physical was performing experiments to study the velociyt and wavelength of the electron. In one case, the electron was accelerated through a potential differences of 1 KV and in second case it was accelerated through a potential difference of 2KV.
The velocity acquired by the electron will be
View SolutionCl−O∣∣C−OC2H5−(1)'X'RMgX−−−−−−−−−→(2)NH4Cl3∘alcohol
View SolutionTreatment of (+)− glucose with HIO4 gives results that confirm its aldohexose structure. What product should be formed, and how much HIO4 should be consumed.
View SolutionA hydrogen like atom (atomic number Z) is in a higher excited satte of quantum number n .This excited atom can make a transition to the first excited state by succesively emitting two photon of energies 10.20 eV and 17.00 eV .Alternatively, the atom from the same excited state can make a transition to the second excited state by successively emitting twio photon of energy 4.25 ev and 5.95 eV Determine the followings: The atom during transition from n = 1 to n = 2 emit radiation in the region of
View SolutionThe ionization potential of H-atom is 13.6 V. The H-atoms in ground state are excited by mono chromatic radiations of photon energy 12.09 ev. Then the number of spectral lines emitted by the excited atoms, will be
View SolutionExcited atoms emit radiations consisting of only certain discrete frequencise or wavelengths. In spectroscopy it is often more convenient to use freuquencies and wave numbers are proportional ot energy and spectroscopy involves transitions between different energy levels . The line spectrum shown by a single electron excited atom (a finger print of an atom) can be given as 1/(lambda)=barvR_(H).Z^(2)[(1)/(n_(1)^(2))-(1)/(n_(2)^(2))] where Z is atomic number of single electron atom and n_(1),n_(2) are integers and if n_(2)gt n_(1), then emission spectrum is noticed and if n_(2)ltn_(1), then absorption spectrum is noticed. Every line in spectrum can be represented as a difference of two terms (R_(H)Z^(2))/(n_(1)^(2)) "and"(R_(H)Z^(2))/(n_(2)^(2)). The ratio of wavelength for II line fo Balmer series and I line of Lyman series for H-like species is :
View SolutionExcited atoms emit radiations consisting of only certain discrete frequencise or wavelengths. In spectroscopy it is often more convenient to use freuquencies and wave numbers are proportional ot energy and spectroscopy involves transitions between different energy levels . The line spectrum shown by a single electron excited atom (a finger print of an atom) can be given as 1/(lambda)=barvR_(H).Z^(2)[(1)/(n_(1)^(2))-(1)/(n_(2)^(2))] where Z is atomic number of single electron atom and n_(1),n_(2) are integers and if n_(2)gt n_(1), then emission spectrum is noticed and if n_(2)ltn_(1), then absorption spectrum is noticed. Every line in spectrum can be represented as a difference of two terms (R_(H)Z^(2))/(n_(1)^(2)) "and"(R_(H)Z^(2))/(n_(2)^(2)). H-atoms in ground state (13.6 eV) are excited by monochromatic radiations of photon of energy 12.09 eV. The number of different spectral lines emitted in H-atoms will be:
View SolutionZ परमाणु-क्रमांक वाले परमाणु की किसी दी गई कक्षा में इलेक्ट्रॉन की ऊर्जा समानुपाती होता है-
View SolutionIf the electron in a hydrogen atom jumps from an orbit with level n_(1)=2 an orbit with level n_(2)=1 . The emitted radiation has a wavelength given by
View SolutionWhat will be the number of spectral lines(N) observed if an electron undergoes transition from n_(2) excited level to n_(1) excited level in an atom of hydrogen-
View SolutionThe ionization energy of the electron in the hydrogen atom in its ground state is 13.6 eV. The atoms are excited to higher energy levels to emit radiations of 6 wavelengths. Maximum wavelength of emitted radiation corresponds to the transition between
View SolutionExcited atoms emits radiations consisting of only certain discrete frequencies or wavelengths. In spectroscopy it is often more convenient to use frequencies or wave numbers than are proportional to energy and spectroscopy involves transitions between energy levels. The line spectrum shown by and mono electronic excited atom (a finger pring of an atom) can be given as
1λ=¯v=RAZ2[1n21−1n22]
When Z is atomic number of mono electronic atom and n1,n2 are integers and if n2>n1, then emission spectrum is noticed and if n2<n1, then absorptioni spectrum is noticed. Every line in spectrum can be represented as differences of two terms RAZ2n21 and RAZ2n22
H-atom in ground state (13.6eV) are excited by monochromatic radiation of photon of energy 12.1 eV. The number of spectral lines emitted in H-atom will be
View SolutionExcited atoms emit radiations consisting of only certain discrete frequencise or wavelengths. In spectroscopy it is often more convenient to use freuquencies and wave numbers are proportional ot energy and spectroscopy involves transitions between different energy levels . The line spectrum shown by a single electron excited atom (a finger print of an atom) can be given as
1λ=¯vRH.Z2[1n21−1n22]
where Z is atomic number of single electron atom and n1,n2 are integers and if n2>n1, then emission spectrum is noticed and if n2<n1, then absorption spectrum is noticed.
Every line in spectrum can be represented as a difference of two terms RHZ2n21andRHZ2n22.
The ratio of wavelength for II line fo Balmer series and I line of Lyman series for H-like species is :
View SolutionIf the electron in a hydrogen atom jumps from an orbit with level n2=3 to orbit with level n1=2, the emitted radiation has a wavelength given by .
View Solution

