The standard molar enthalpies of formation of cyclohexane (I) and benzene(I) at 25∘C are −156 and +49KJmol−1 respectivelyl. The standard enthalpy of hydrogentation of cyclohexene (I) at 25∘ is −119KJmol−1. Use these data to estimate the magnitude of the resonance energy of benzene.
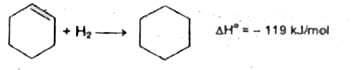
This implies that the generation of one double bond in cyclohexane requires 119 kJ/mol of enthalpy. To calculate the resonance energy we may proceed as follows:
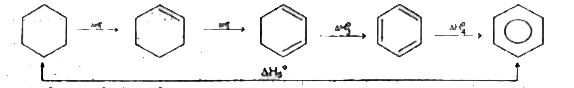
ΔH∘1=ΔH∘2=ΔH∘3=119 kJ/mol.
ΔH∘4 = resonance energy
ΔH∘5=ΔH∘r(Benzene)−ΔH∘f(Cyclohexane)
=49−(−156)
According to Hess.s law
ΔH∘4=ΔH∘5−(ΔH∘1+ΔH∘2+ΔH∘3)
=205 - 3 ×119
=−152 kJ/mole
Similar Questions
25”∘C पर हाइड्रोजन, साइक्लोहेक्सीन (C6H10) तथा साइक्लोहेक्सेन (C6H12) की मानक दहन कमाएँ क्रमशः -241,-3800 तथा -3920 किलोजूलामोल हैं। साइक्लोहेक्सीन के हाइड्रोजनीकरण की ऊष्मा की गणना कीजिए।
View SolutionThe standard molar enthalpies of formation of cyclohexane (l) and benzene (l) at 25∘Care−156and+49kJmol−1 respectively. The standard enthalpy of hydrogenation of cyclohexene (l) at 25∘C is −119kJmol−1. Use these data to estimate the magnitude of the resonance energy of benzene.
View SolutionThe standard enthalpy of hydrogenation of cyclohexene is119 kJmol−1 . If enthalpies for formation of cyclohexane and benzene at 25∘C are respectively -156 and + 49 kJ mol−1 , estimate the magnitude of resonance energy of benzene.
View SolutionIf enthalpy of hydrogenation of cyclohexene is [-x kJ mol^-1] and resonance energy of benzene is [-y kJ mol^-1] then the enthalpy of hydrogenation of benzene will be
View SolutionThe standard molar enthalpies of formation of cyclohexane (l) and benzene (l) at 25^(@)C are -156 and +49 kJ mol^(-1) respectively. The standard enthalpy of hydrogenation of cyclohexene (l) at 25^(@)C is -119 kJ mol^(-1) . Use these data to estimate the magnitude of the resonance energy of benzene.
View SolutionThe standard molar enthalpies of formation of cyclohexane (l) and benzene (l) at 25∘C are −156 and +49kJmol−1, respectively. The standard enthaly of hydrogenation of cyclohenxene (l) at 25∘C is −119kJmol−1 Use this data to estimate the magnitude of the resonance enegry of benzene.
View SolutionIf enthalpy of hydrogenation of cyclohexene is [−xkJmol−1] and resonance energy of benzene is [−ykJmol−1] then the enthalpy of hydrogenation of benzene will be
View Solution

