Calculate enthalpy change of the following reaction :
CH2=CH2(g)+H2(g)→CH3−CH3(g)
The bond energy of C - H, C - C, C = C, H - H are 414, 615 and 436 kJ mol−1 respectively.
−100kJ
−125kJ
−150kJ
−175kJ
The correct Answer is:B
Step by step video, text & image solution for Calculate enthalpy change of the following reaction : CH_(2) = CH_(2)(g) + H_(2)(g) rarr CH_(3) - CH_(3)(g) The bond energy of C - H, C - C, C = C, H - H are 414, 615 and 436 kJ mol^(-1) respectively. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL-I (REASONING TYPE QUESTIONS)
Explore 2 Videos
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL-II
Explore 25 Videos
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL -2
Explore 15 Videos
- TEST PAPERSBook:FIITJEEChapter:TEST PAPERSExercise:CHEMISTRY
Explore 747 Videos
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBook:FIITJEEChapter:TRANSITION ELEMENTS & COORDINATION COMPOUNDSExercise:MATCHIG LIST TYPE QUESTIONS
Explore 1 Video
Similar Questions
Statement-1: The heat absorbed during the isothermal expansion of an ideal gas against vaccum is zero.
Statement -2: The vaccum occupied by the molecules of an ideal gas is zero.
View SolutionThe intensive property/properties is/are
View SolutionFor the reaction, 2CO+O2→2CO2,ΔH=−560kJ. Two moles of CO and one mole of O2 are taken in a container of volume 1 L. They completely form two moles of CO2, the gases deviate appreciably from ideal behaviour. If the pressure in the vessel changes from 70 to 40 atm, find the magnitude (absolute value) of ΔU at 500 K.
View SolutionFor a 3s-orbital
Φ(3s)=1a√3(1a0)3/2(6−6σ+σ2)∈−σ/2
where σ=2rZ3aσ
What is the maximum radial distance of node from nucleus?
View Solution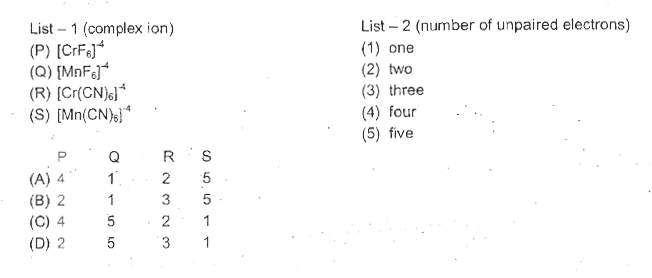
View SolutionCalculate the enthalpy of the following reaction: H_(2)C =CH_(2)(g) +H_(2)(g) rarr CH_(3)-CH_(3)(g) The bond enegries of C-H, C-C,C=C , and H-H are 99,83,147 ,and 104kcal respectively.
View SolutionCalculate enthalpy change of the following reaction : H_(2)C=CH_(2(g))+H_(2(g)) rarr H_(3)C-CH_(3(g)) The bond energy of C-H,C-C,C=C,H-H are 414,347,615 and 435k J mol^(-1) respectively.
View SolutionIf a 298K the bond energies of C-H,C-C,C=C and H-H bonds are respectivly 414, 347, 615KJmol^(-1) , the vlaue of enthalpy change for the reaction H_(2)C=CH_(2)(g)+H_(2)(g)+H_(2)(g)rarrH_(3)C-CH_(3)(g) at 298K will be
View SolutionCalculate DeltaH^(@) ("in" kJmol^(-1)) for the reaction CH_(2)Cl_(2)(g)rarrC(g)+2H(g)+2Cl(g). The average bond enthalpie of C-H and C-C1 bonds are 414 kJ mol^(-1) .
View SolutionCalculate the enthalpy for the following reaction using the given bond energies (kJ/mol) : (C-H=414, H-O =463, H-Cl = 431, C-Cl=326, C-O =335) CH_(3)-OH(g)+HClrarrCH_(3)-Cl(g)+H_(2)O(g)
View SolutionCalculate the enthalpy of the following reaction : H_(2)C = CH_(2)(g) + H_(2)(g) rarr H_(3)C - CH_(3)(g) The bond energies of C-H,C-C,C=C and H-H are 414,347,615 and 435 kJ mol^(-1) respectively
View SolutionCalculate enthalpy change of the following reaction :
CH2=CH2(g)+H2(g)→CH3−CH3(g)
The bond energy of C - H, C - C, C = C, H - H are 414, 615 and 436 kJ mol−1 respectively.
View Solutionनिम्न अभिक्रिया की एन्थैल्पी की गणना कीजिये ।
CH2=CH2(g)+H2(g)→CH3−CH3(g)
C−H,C−C,C=C तथा H−H आबंधो की आबन्ध ऊर्जाऐ क्रमश : 414,347,615 और 425kJmol−1 हैं।
View SolutionCH_(2)=CH_(2)(g)+H_(2)(g) to CH_(3) to CH_(3)(g) The heat of reaction is [bond energy of C=C=80 kcal, C=C=145 kcal, C-H=98 kcal, H-H=103 kcal]
View SolutionCalculate enthalpy change of the following reaction :
CH2=CH2(g)+H2(g)→CH3−CH3(g)
The bond energy of C - H, C - C, C = C, H - H are 414, 615 and 436 kJ mol−1 respectively.
View Solution
