Video Solution
Text Solution
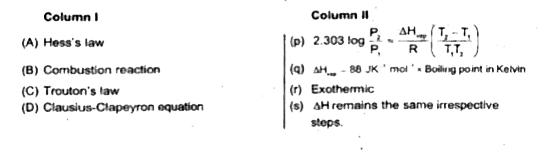
(A)→(Q), Here.s law states that enthalpy change in a reaction remains the same whether the reaction takes placce in one step or in several steps.
(B)→(R), Combustion reactions are exothermic.
(C)→(Q),ΔHvapBoiling point in K=88JK−1mol−1
(D)→(P),2.303logP2P1=ΔHvapRT2−T1T1T2
In a Clausius -Clapeyron equation.
|
Topper's Solved these Questions
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:(MATCHING LIST TYPE QUESTIONS)
Explore 5 Videos
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:(MATCHING TYPE SINGLE CORRECT QUESTION)
Explore 3 Videos
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:ASSIGNMENT PROBLEMS (OBJECTIVE) COMPREHENSION -III
Explore 6 Videos
- TEST PAPERSBook:FIITJEEChapter:TEST PAPERSExercise:CHEMISTRY
Explore 747 Videos
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBook:FIITJEEChapter:TRANSITION ELEMENTS & COORDINATION COMPOUNDSExercise:MATCHIG LIST TYPE QUESTIONS
Explore 1 Video