The metal-insoluble salt electrode consists of a metal M coated with a porous insoluble salt MX In a solution of X−, A good example is Ihe silver, silver-chloride electrode for which the half-cell reaction is AgCI(s)+e⇔Ag(s)+CI(aq), where, the reduction of sold silver chloride produces solid silver and releases chloride ion into solute for the cell
M(s)∣∣M2+(aq)∣∣∣∣Ag+(aq)∣∣Ag(s) E∘M/M2+=+2.37VandE∘Ag/Ag=+0.80V
If cell reaction is in equilibrium stage, value of logarithms of equilibrium constant is
1.0745
107.45
1074.5
10.745
The correct Answer is:B
Topper's Solved these Questions
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:(Comprehension -V)
Explore 8 Videos
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:Matrix Match Type Questions
Explore 8 Videos
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:(Comprehension -III)
Explore 2 Videos
- CONCEPT OF ACIDS AND BASESBook:FIITJEEChapter:CONCEPT OF ACIDS AND BASESExercise:SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 4 Videos
- ELECTROPHILIC AROMATIC SUBSTITUTIONBook:FIITJEEChapter:ELECTROPHILIC AROMATIC SUBSTITUTIONExercise:NUMERICAL BASED QUESTIONS INDICATED BY DIGITAL INTERGER
Explore 1 Video
Similar Questions
Cell voltages and half-cell potentials are often compeared under standard conditions called the standard state at a specified temperature (298K). The standard state requires all reagents to be pure with all gases at unit fugacity, all ions at unit activity and all electrical connections between half-cells to be made with platinum. Suppose following are half cell reaction, with respective emf (s) are -0.25 V and -0.3V.
Cell reaction I Pt(H2)∣HCl1 atm
Cell reaction II Pt(H2)∣H2SO41 atm
pH value of cell reaction I is
View SolutionMatch the Column -I with Column -II:

View SolutionFollowing two graphs are based on the conductometric titration of acid-base reaction. Answer the questions given at the end of it.
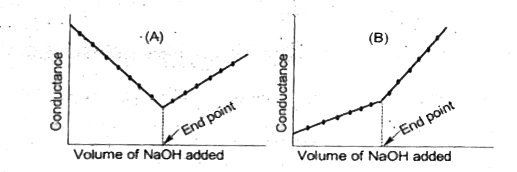
Select correct statement,
View Solution100 mL of 0.2 N HCI is added to 100 mL of 0.18 N NaOH and whole volume is made one litre. What will be the pH of resulting solution.
View SolutionPhN+2BF−4PhNO2−−−−→ΔA
If the molecular weight of product A is x, x4?
View SolutionDerive Nernst equation for the cell.
Ni(s)∣∣Ni2+(aq.0.1M)∣∣∣∣Ag+(aq.0.1M)∣∣Ag(s) and also find its cell potential. Given :
E∘Ag+/Ag=0.80 volt and E∘Ni2+/Ni=−0.25 volt
View Solutionकॉपर और सिल्वर के बीच एक सेल निर्मित किया गया है-
Cu(S)Cu2+(aq)∣∣Ag+(aq)/Ag(s)
अगर दो अर्ध्दसेल मानक अवस्था में कार्य करते है तो सेल के EMF की गणना कीजिए।
ECu2+/Cu=+0.34V
EAg+/Ag=0.80V
View SolutionFor the cell reaction,
Mg∣∣Mg2+(aq.)∣∣∣∣Ag+(aq.)∣∣Ag
calculate the equilibrium constant at 25∘C and maximum work that can be obtained by operating the cell.
E∘Mg2+/Mg=−2.37 volt and E∘Ag+/Ag=+0.80 volt
View SolutionCu(s)∣∣Cu+2(aq,10−3)∣∣∣∣Ag+(10−5M)∣∣Ag(s)
if ECu+2/Cu=0.34V ltBrgt EAg+/Ag=+0.80V
Ecell will be :-
View SolutionCalculate the concentration of silver ions in the cell constructed by using 0.1 M concentration of Cu^(2+) and Ag^(+) ions. Cu and Ag metals are used as electrodes. The cell potential is 0.422 V. [E _(Ag^(2+)//Ag)=0.80V,E_(Cu^(2+)//Cu)=+0.34V]
View SolutionFollowing cell is set up between copper and silver electrodes : Cu|Cu^(2)(aq)||Ag^(+)(aq)|(Ag) if its two half cells work under standard condition calculate the e.m.f of the cell ["Given" E_(Cu^(2+)/Cu)^(@)=0.34 "volt" E_(Ag^(+)/Ag=+0.80 "volt")^(@)]
View SolutionFor the standard cell Cu(s)∣∣Cu2+(aq)∣∣∣∣Ag+(aq)∣∣Ag(s). [ECu2+Cu=0.3VandE0Ag+Ag=0.80V]
Identify the cathode and the anode as the current is drawn from the cell.
View SolutionFor the standard cell Cu(s)∣∣Cu2+(aq)∣∣∣∣Ag+(aq)∣∣Ag(s). [ECu2+Cu=0.3VandE0Ag+Ag=0.80V]
Write the reaction taking place at the electrodes.
View SolutionA silver electrode is immersed in a 0.1 (M) AgNO3 solution at 25∘C. If AgNO3 dissociates almost completely in the solution, then determine the potential of the silver electrode.
Given : E∘Ag+∣Ag=0.80V.
View SolutionIs 1.0 M H^+ solution under H_2SO_4 at 1.0 atm capable of oxidising silver metal in the presence of 1.0 M Ag^+ ion E_(Ag^+|Ag)^0=0.80V, E_(H^+|H_2(Pt))^0=0.0V
View SolutionThe metal-insoluble salt electrode consists of a metal M coated with a porous insoluble salt MX In a solution of X^(-), A good example is Ihe silver, silver-chloride electrode for which the half-cell reaction is AgCI(s)+e Leftrightarrow Ag(s) + CI (aq), where, the reduction of sold silver chloride produces solid silver and releases chloride ion into solute for the cell M(s)|M^(2+) (aq)||Ag^(+) (aq)|Ag(s)" "E_(M//M^(2+))^(@)=+2.37V and E_(Ag//Ag)^(@)=+0.80V Emf of given cell, when concentration of M^(2+) ion and concentration of Ag^(+) ion both are 0.01 M
View Solution

