The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
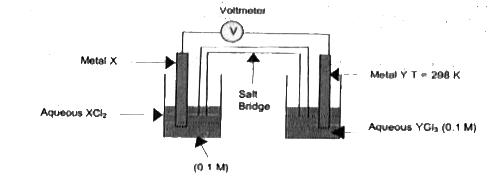
Cell reaction is spontaneous:
in the given condition
if half-cells are interchanged
if [XCI2]=0.01Mand[Ycl3]=1M
if solution in both half-cells are diluted 100 times
The correct Answer is:B
Step by step video, text & image solution for The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl_(2) and YCl_(3). Given that 3X(s)+2Y^(3+) (aq) to 3X^(2)(aq)+2Y(s) epsi_("cell") gt 0 Cell reaction is spontaneous: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:Matrix Match Type Questions
Explore 8 Videos
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:Matcing Type Single Correct Questions
Explore 3 Videos
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:(Comprehension -IV)
Explore 4 Videos
- CONCEPT OF ACIDS AND BASESBook:FIITJEEChapter:CONCEPT OF ACIDS AND BASESExercise:SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 4 Videos
- ELECTROPHILIC AROMATIC SUBSTITUTIONBook:FIITJEEChapter:ELECTROPHILIC AROMATIC SUBSTITUTIONExercise:NUMERICAL BASED QUESTIONS INDICATED BY DIGITAL INTERGER
Explore 1 Video
Similar Questions
Write the cell reaction for the electrochemical cell represented as
Zn(s)∣∣Zn2+(aq)∣∣∣∣Cu2+(aq)∣∣Cu(s)
View SolutionThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
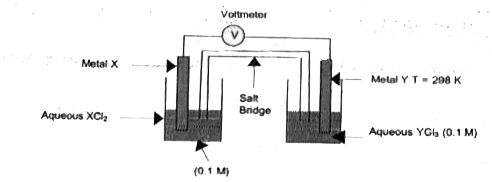
E∘X/X2+=0.20v,E∘Y/Y3+=0.40V the E∘cell of the above designed cell is
View Solution
Knowledge Check
The following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
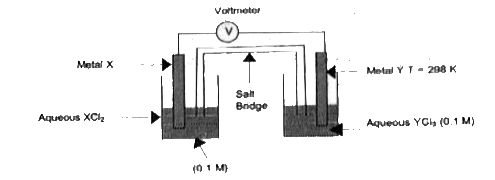
if salt-bridge is removed:AE∘X/X2+ changesBE∘Y/Y3+ changesCEcell drops to zeroDEcell increasesThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
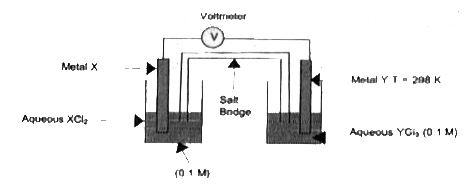
Ecell of the above designed cell is if standard emf od cell is -0.20 V.A(0.20+0.05912)VB(0.20−0.05913)VC(−0.20+0.05916)VD+2.0VThe following diagram shown as electrochemical cell in which the respective half-cells contains aqueous 1.0M solutions of the salts XCl2andYCl3. Given that
3X(s)+2Y3+(aq)→3X2(aq)+2Y(s)εcell>0
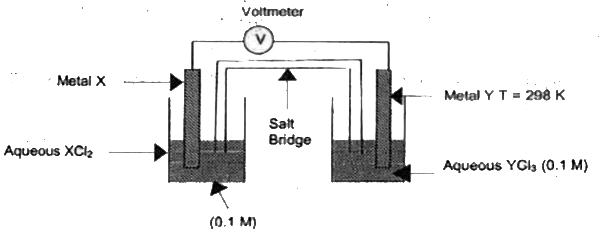
Which of the following statements is correct?
I. The electrode made from metal X has positive polarity
II. Electrode Y Is the anode
II. Tho flow of electrons is from Y to X
IV. The reaction at electrode X is an oxidation
V. The salt bridge would most likely contain silver nitrateAonly IBonly I & IVConly VDall except V

The following diagram shows the setup of an electrochemical cell in which the respective half cells contain aqueous 1.0 M solutions of the salts XCl2 and YCl3. Given that
3X(s)+2Y3+(aq)→3X2+(aq)+2Y(s)EcellL<0 Which of the following statements is correct?
View SolutionIn the reaction ,
Cu(s)+2Ag+(aq)→Cu2+(aq)+2Ag(s), the reduction half cell reaction is
View SolutionConsider an electrochemical cell in which the following reaction occurs and predict which changes will decrease the cell voltage :
Fe2+(aq)+Ag+(aq)→Ag(s)+Fe3+(aq)
View SolutionIn a galvanic cell in which the following spontaneous reaction takes place, what process occurs at the cathode?
3Ce4+(aq)+Cr(s)→3Ce3+(aq)+Cr3+(aq)
View SolutionIn the reaction
Cu(s)+2Ag+(aq)→Cu2+(aq)+2Ag(s)
the reduction half-cell reaction is:-
View Solution
