Which of the following process is used in the extractive metallurgy of magnesium ?
fused salt electrolysis
Self reduction
aqueous solution electrolysis
thermite reduction
The correct Answer is:A
Topper's Solved these Questions
- ORES AND METALLURGYBOOK - FIITJEECHAPTER - ORES AND METALLURGYEXERCISE - REASONING TYPE QUESTIONS
13 Videos
- ORES AND METALLURGYBOOK - FIITJEECHAPTER - ORES AND METALLURGYEXERCISE - COMPREHENSION
9 Videos
- ORES AND METALLURGYBOOK - FIITJEECHAPTER - ORES AND METALLURGYEXERCISE - SOLVED PROBLEMS (SUBJECTIVE)
10 Videos
- NUCLEIC ACID AND VITAMIN BOOK - FIITJEECHAPTER - NUCLEIC ACID AND VITAMIN EXERCISE - ASSIGNMENT PROBLEMS (OBJECTIVE)
15 Videos
- PERIODIC PROTERTIES OF ELEMENTSBOOK - FIITJEECHAPTER - PERIODIC PROTERTIES OF ELEMENTSEXERCISE - COMPREHENSION
13 Videos
Similar Questions
Statement - 1 : The alkali metals can form ionic hydrides which contain the hydride ion H− and
Statement - 2 : The alkali metals have low electronegativity, their hydrides conduct electricity when fused and liberate hydrogen at the anode.
View SolutionExtraction of aluminum can be understood by
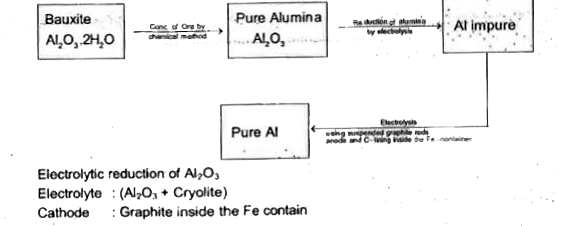
The purpose of adding cryolite is :
View SolutionComplete the following reactions :
(a) AI(OH)−4+CO2→?+?+?
(b) Ag++Na2S2O3→?+?
(c) MnO−4+H2SΔ−−→?+?
View SolutionVitamin C is
View SolutionBohr's periodic table has seven horizontal rows called periods and eighteen vertical columns called groups. Alternatively, the elements of the Bohr's table are divided into four blocks namely S- and f-blocks. Group, period and block of given element can be predicted with the help of its electronic configuration.
(I) The principle quantum number of valence shell refers to period of elements
(II) The nature of subshell (S. p or d) having last electron refers the block to which the element belongs.
(III) The number of electrons present in outermost or penultimate shell predicts the group of element.
For s-block, the group number = No. of valence electron
For p-block, the group number =10+ numbers of valence electron
For d-block, the group number =2+(n−1)d electrons
The element of atomic number 36 belongs to
View SolutionWhich of the following process is used in the extractive metallurgy of magnesium ?
View SolutionWhich of the following processes is used in the extractive metallurgy of magnesium ?
View SolutionWhich of the following processes is used in the extractive metallurgy of magnesium ?
View SolutionWhich of the following processes is used in the extractive metallurgy of magnesium ?
View SolutionWhich of the following process is used in the extractive metallurgy of magnesium ?
View Solutionमैग्नेशियम के निष्कर्षण की धातुकार्मिकी में निम्न में से कौन-सा प्रक्रम प्रयुक्त होता है?
View SolutionWhich of the following process is used in the extraction of metallurgy of magnesium ?
View Solutionwhich of the following process is used in the extractive metallurgy of magnesium?
View SolutionWhich of the following process is used in the extractive metallurgy of magnesium ?
View SolutionWhich of the following process is used in the extraction of metallurgy of magnesium ?
View Solution

