Highly pure dilute solution of sodium in liquid ammonia
A
shows blue colour
B
exhibits electrical conductivity
C
produces sodium amide
D
produces hydrogen gas
Text Solution
The correct Answer is:A, B
|
Topper's Solved these Questions
- ORES AND METALLURGYBook:FIITJEEChapter:ORES AND METALLURGYExercise:COMPREHENSION - I
Explore 4 Videos
- ORES AND METALLURGYBook:FIITJEEChapter:ORES AND METALLURGYExercise:COMPREHENSION - II
Explore 2 Videos
- ORES AND METALLURGYBook:FIITJEEChapter:ORES AND METALLURGYExercise:ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL - I
Explore 50 Videos
- NUCLEIC ACID AND VITAMIN Book:FIITJEEChapter:NUCLEIC ACID AND VITAMIN Exercise:ASSIGNMENT PROBLEMS (OBJECTIVE)
Explore 15 Videos
- PERIODIC PROTERTIES OF ELEMENTSBook:FIITJEEChapter:PERIODIC PROTERTIES OF ELEMENTSExercise:COMPREHENSION
Explore 13 Videos
Similar Questions
Knowledge Check
Highly pure dilute solution of sodium in ammonia
AShows blue colouration due to solvated electronsBShows electrical conductivity due to both solvated electrons and solvated sodium ionsCShows red colouration due to solvated electrons but a bad conductor or electricityDProduces hydrogen gas or carbonateFreshly prepared pure dilute solution of sodium in liquid ammonia:
Ashows copper-bronze colourBoccupy large volume than that from the sum of the volume of Na and NH3 (l).Creduces the Geh4to GeH−2Dproduces sodium amide and hydrogen gas with rusty iron wire.Highly pure dilute solution of sodium in ammonia :
Ashows blue colouration due to solvated electronsBshows electrical conductivity due to both solvated electrons as well as solvated sodium ionsCshows red colouration due to solvated electrons but a bad conductor of electricityDproduces hydrogen gas or carbonate
Which of the following statements is not ture about the dilute solutions of alkali metals in liquid ammonia ?
View Solution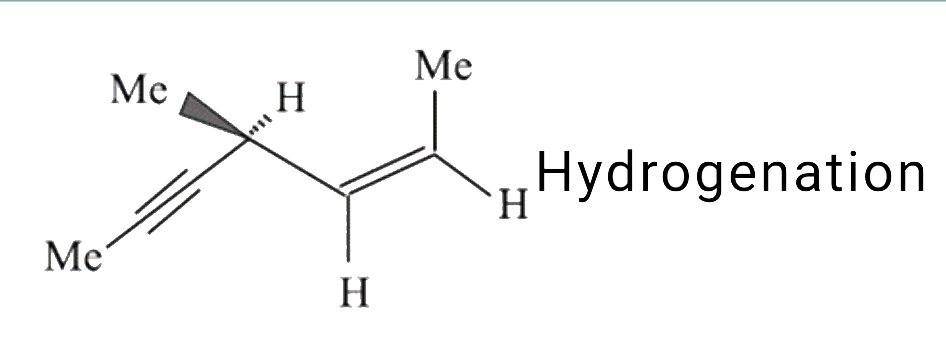 Hydrogenation of the above compound in the presence of sodium in liquid ammonia gives -
Hydrogenation of the above compound in the presence of sodium in liquid ammonia gives -
View Solution..................... is the dilute solution of liquor ammonia.
View SolutionWhich of the following statements is not ture about the dilute solutions of alkali metals in liquid ammonia ?
View Solution Hydrogenation of the above compound in the presence of sodium in liquid ammonia gives -
Hydrogenation of the above compound in the presence of sodium in liquid ammonia gives -
View Solution

