Which of the following is the strongest base?
The correct Answer is:C
To determine which of the given options is the strongest base, we will analyze each option based on the presence of electron-donating groups and their effects on the basicity of the compounds.
1. Identify the Compounds: We have four options, each representing a different compound. We need to analyze the structure and substituents of each compound to determine their basicity.
2. Analyze Option 1:
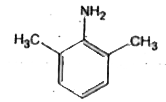
- This compound is a 1,5-di-substituted aniline with two methyl groups attached to the ortho positions of the benzene ring.
- Methyl groups are electron-donating through the +I effect, which increases the electron density on the benzene ring and enhances the basicity of aniline.
- Therefore, this compound has increased basicity due to the presence of two methyl groups.
3. Analyze Option 2:
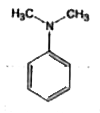
- This compound also has a nitrogen atom with a lone pair and two substituents: a methyl group and another group (assumed to be electron-withdrawing).
- The lone pair on nitrogen can participate in resonance with the benzene ring, but the presence of the electron-withdrawing group can decrease the overall electron density on the nitrogen.
- Thus, while this compound is basic, it is less basic than Option 1 due to the electron-withdrawing effect.
4. Analyze Option 3:
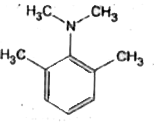
- This compound has two methyl groups on the nitrogen and is also ortho to the benzene ring.
- The presence of two electron-donating groups (methyl) increases the electron density on the nitrogen significantly.
- This compound has the highest electron density on the nitrogen due to both resonance and the +I effect from the methyl groups, making it the strongest base among the options.
5. Analyze Option 4:
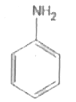
- This is a simple aniline without any substituents.
- While aniline is basic, it lacks any electron-donating groups to enhance its basicity further.
- Therefore, this compound is the least basic among the four options.
6. Conclusion:
- After analyzing all four options, we conclude that Option 3, with the highest electron density due to the presence of two methyl groups, is the strongest base.
Final Answer:
Option 3 is the strongest base.
---
- This compound is a 1,5-di-substituted aniline with two methyl groups attached to the ortho positions of the benzene ring.
- Methyl groups are electron-donating through the +I effect, which increases the electron density on the benzene ring and enhances the basicity of aniline.
- Therefore, this compound has increased basicity due to the presence of two methyl groups.
- This compound also has a nitrogen atom with a lone pair and two substituents: a methyl group and another group (assumed to be electron-withdrawing).
- The lone pair on nitrogen can participate in resonance with the benzene ring, but the presence of the electron-withdrawing group can decrease the overall electron density on the nitrogen.
- Thus, while this compound is basic, it is less basic than Option 1 due to the electron-withdrawing effect.
- This compound has two methyl groups on the nitrogen and is also ortho to the benzene ring.
- The presence of two electron-donating groups (methyl) increases the electron density on the nitrogen significantly.
- This compound has the highest electron density on the nitrogen due to both resonance and the +I effect from the methyl groups, making it the strongest base among the options.
- This is a simple aniline without any substituents.
- While aniline is basic, it lacks any electron-donating groups to enhance its basicity further.
- Therefore, this compound is the least basic among the four options.
- After analyzing all four options, we conclude that Option 3, with the highest electron density due to the presence of two methyl groups, is the strongest base.
Option 3 is the strongest base.
Topper's Solved these Questions
- CONCEPT OF ACIDS AND BASESBook:FIITJEEChapter:CONCEPT OF ACIDS AND BASESExercise:ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-I REASONING TYPE QUESTONS
Explore 2 Videos
- CONCEPT OF ACIDS AND BASESBook:FIITJEEChapter:CONCEPT OF ACIDS AND BASESExercise:ASSIGNEMENT PROBLEMS (OBJECTIVE)Level-II
Explore 15 Videos
- CONCEPT OF ACIDS AND BASESBook:FIITJEEChapter:CONCEPT OF ACIDS AND BASESExercise:ASSIGNMENT PROBLEMS (SUBJECTIVE)Level-II
Explore 9 Videos
- CHEMISTRY IN EVERY DAY LIFE Book:FIITJEEChapter:CHEMISTRY IN EVERY DAY LIFE Exercise:ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)
Explore 5 Videos
- ELECTROCHEMISTRYBook:FIITJEEChapter:ELECTROCHEMISTRYExercise:Matcing Type Single Correct Questions
Explore 3 Videos
Similar Questions
Knowledge Check
Which of the following is a strongest base ?
ANH3BCH3NH2C(CH3)2NHD(CH3)2NWhich of the following is a strongest base ?
AAmmonium hydroxideBSodium hydroxideCMagnesium hydroxideDCopper hydroxideWhich among the following is the strongest base?
ABe(OH)2BMg(OH)2CBa(OH)2DSr(OH)2
Which one of the following is the strongest base
View SolutionWhich of the following is the strongest Bronsted base?
View SolutionWhich of the following is the strongest Bronsted base ?
View SolutionWhich one of the following is the strongest base in aqueous solution?
View SolutionWhich one of the following is the strongest base in aqueous solution ?
View Solution
