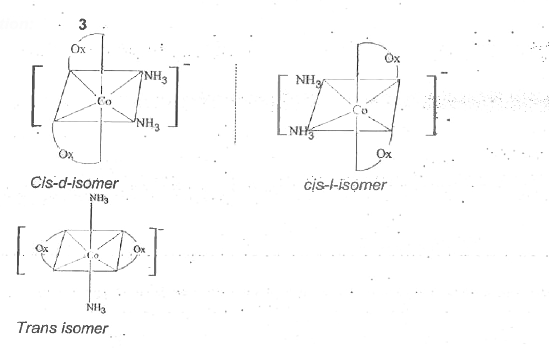
What is the number of possible isomer of an octahedral complex : [Co(C2O4)2(NH3)2]
Text Solution
|
Topper's Solved these Questions
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBook:FIITJEEChapter:TRANSITION ELEMENTS & COORDINATION COMPOUNDSExercise:MATCHING TYPE SINGLE CORRECT QUESTION
Explore 9 Videos
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBook:FIITJEEChapter:TRANSITION ELEMENTS & COORDINATION COMPOUNDSExercise:Exercise
Explore 14 Videos
- TRANSITION ELEMENTS & COORDINATION COMPOUNDSBook:FIITJEEChapter:TRANSITION ELEMENTS & COORDINATION COMPOUNDSExercise: REASONING TYPE QUESTIONS
Explore 4 Videos
- THERMODYNAMICS AND THERMOCHEMISTRY Book:FIITJEEChapter:THERMODYNAMICS AND THERMOCHEMISTRY Exercise:SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 5 Videos
Similar Questions
Knowledge Check
The number of possible isomers of an octahedral complex [Co(C2O4)2(NH3)2] is
A1B2C3D4The number of possible of an octahedral complex [Co(C2O4)2(NH3)2]− is
A1B2C3D4What is the number of possible isomers for the octahedral complex [Co(NH3)2(C2O4)2] ?
A1B2C3D4
The number of stereo isomers of the compound is [Co(C2O4)(NH3)2(NO2)2]
View SolutionWrite all geometric isomers for an octahedral complex [MCI2(NH3)4] .
View SolutionWrite all geometric isomers for an octahedral complex [MCI2(NH3)4] .
View SolutionThe number of possible isomers for the complex [Co(C2O4)(NH3)2] is
View SolutionThe number of geometrical isomers of the complex [Co(NO2)3(NH3)3] is
View Solution