The deep blue colour obtained on adding K Fe(CN), to ferrous sulphate is due to the formation of
A
Fe[Fe(CN)6]
B
Fe[Fe(CN)6l]2
C
Fe3[Fe(CN)6]4
D
Fe4[Fe(CN)6]3
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
The Prussian blue colour obtained in the Lassaigne' s test for nitrogen is due to the formation of
AIron (II) hexacyanoferrate (III)BIron (III) hexacyanoferrate (II)CIron (III) hexacyanoferrate (III)DSodium hexacyanoferrate (III)The deep blue colour produced on adding excess of ammonia to copper sulphate is due to presence of
ACu(NH3)2+4BCu2+CCu(NH3)2+2DCu(NH3)2+6The prussian blue colour obtained in the Lassaigne's test for nitrogen is due to formation of :
AIron (II) hexa cyano ferrate (II)BIron (III) hexa cyano ferrate (II)CIron (III) hexa cyano ferrate (III)DIron (II) hexa cyano ferrate (III)
Colour of ferrous sulphate crystals
View SolutionChoose the most appropriate options.
The metallic sodium dissolved in liquid ammonia to form a deep blue colour solution.The deep blue colour is due to the formation of
View SolutionThe Lassaigne's solution when heated with ferrous sulphate and acidified with sulphuric acid gave intense blue colour indicating the presence of nitrogen. The blue colour is due to formation of:
View Solution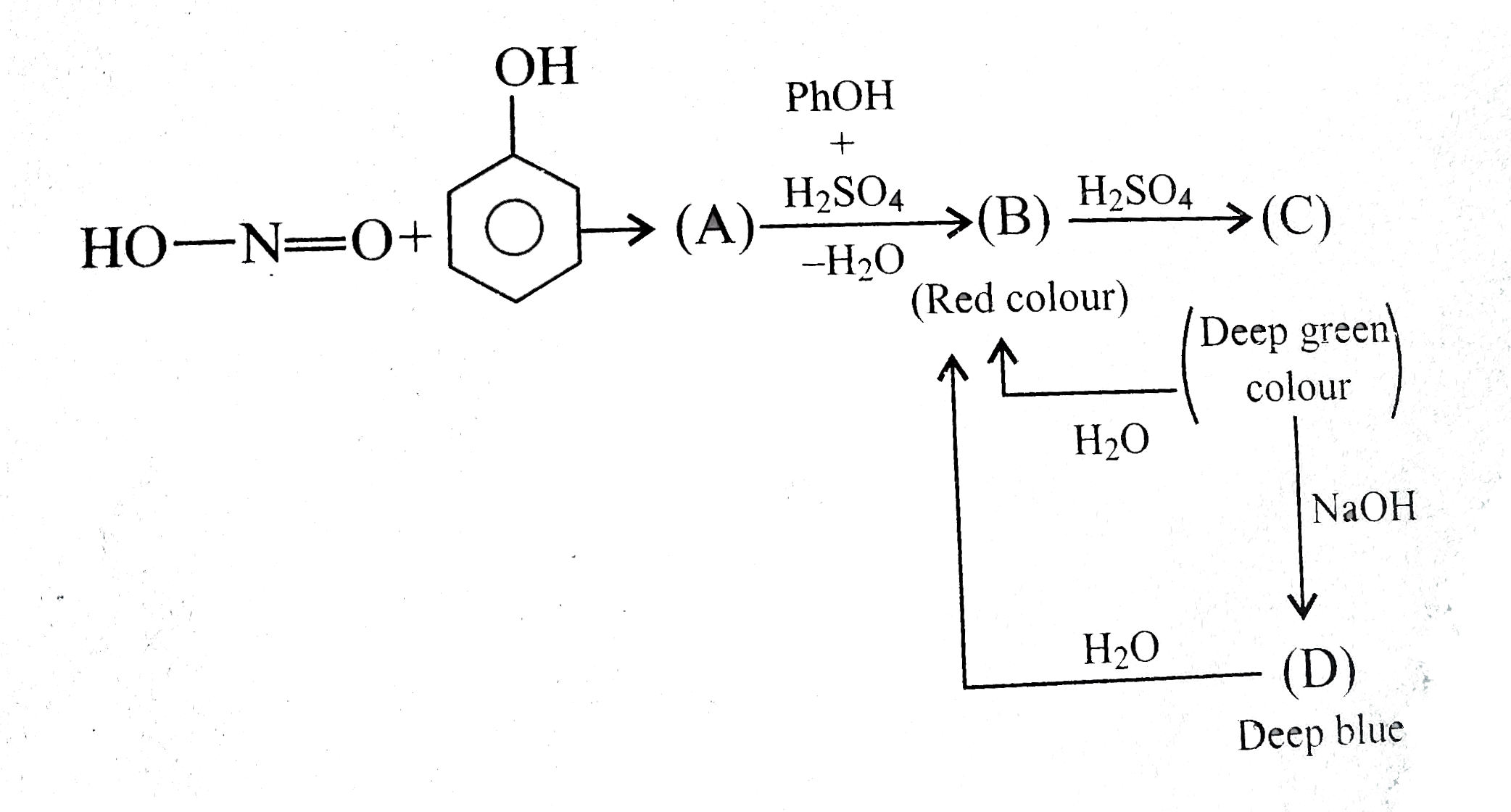
The deep blue colour of compound (D) is due to the formation of:
View SolutionDeep blue colour formed by addition of copper (II) suphate solution to ethylamine is due to formation of
View Solution