The radio-nuclide Th23490 undergoes two successive β- decays followed by one α- decay. The atomic number and the mass number respectively of the resulting radionuclide are:
A
92 and 234
B
94 and 230
C
90 and 230
D
92 and 230
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
The radioactive nuclide .23490Th shows two successive β− decay followed by one α− decay. The atomic number and mass number respectively of the resulting atom is:
A92 and 234B94 and 230C90 and 230D92 and 230The radioactive nuclide .23490Th shows two successive β− decay followed by one α− decay. The atomic number and mass number respectively of the resulting atom is:
A90 and 230B92 and 230C92 and 234D94 and 230The radioactive nuclide 90234Th shows two succesive β-decay followed by one α-decay. The atomic nuimber and mass number respectively of the resulting atom is
A90 and 230B92 and 230C92 and 234D94 and 230
A radioactive element, U23892 undergoes successive radioactive decay and decays into Pb20682 The number of αandβ particles emitted respectively are
View SolutionA radioactive element, U23892 undergoes successive radioactive decay and decays into Pb20682 The number of αandβ particles emitted respectively are
View SolutionA radioactive nucleus A undergoes a series of decays as given below.
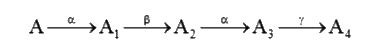
The mass number and atomic number of are 176 and 71 respectively.
Determie the mass and atomic number of A4 and A.
View SolutionIf .92U238 undergoes successively 8α−decays and 6β−decays, then resulting nucleus is.
View SolutionNuclides having some atomic number and same mass number but different rate of decay are called:
View Solution

