For the reaction 2N2O5→4NO2+O2 rate of reaction and rate constant are 1.02×10−4 and 3.4×10−5sec−1 respectively. The concentration of N2O5 at that time will be
1.732mol/l
3mol/l
1.02×10−4mol/l
3.4×105mol/l
The correct Answer is:B
Step by step video, text & image solution for For the reaction 2N_(2)O_(5) rarr 4NO_(2)+O_(2) rate of reaction and rate constant are 1.02 xx 10^(-4) and 3.4 xx 10^(-5) sec^(-1) respectively. The concentration of N_(2)O_(5) at that time will be by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
अभिक्रिया, 2N2O5(g)→4NO2(g)+O2(g) के लिए ______________ ×N2O5 के विलुप्त होने की दर =___________ ×NO2 के सम्भवन की दर।
View Solutionयदि 2N2O5→4NO2+O2 के लिए दर – स्थिरांक 3.0×10−5s−1 है। यदि दर =2.4×10−5molL−1s−1 हो तो N2O5 का molL−1 में सान्द्रता होगा।
View SolutionFor the reaction 2N2O5→4NO2+O2 rate and rate constant are 1.22×10−4and3.4×10−5S−1 respectively then the concentration of N2O5 at that time will be
View SolutionFor the reaction, 2N_(2)O_(5)to4NO_(2)+O_(2) rate and rate constant are 1.02xx10^(-4) M sec^(-1) and 3.4xx10^(-5)sec^(-1) respectively, the concentration of N_(2)O_(5) , at that time will be
View SolutionThe rate constant for the reaction, 2N2O5→4NO2+O2 is 3.0×10−5s−1. If the rate is 2.40×10−5molL−1s−1, then the initial concentration of N2O5 (in molL−1) is
View Solution2N2O5
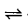 4NO2+O2 પ્રક્રિયા માટે વેગ અને વેગ અચળાંક અનુક્રમે 1.02×10−4 અને 3.4×10−5S−1 છે તો આ સમયે N2O5 ની સાંદ્રતા કેટલી હશે?
4NO2+O2 પ્રક્રિયા માટે વેગ અને વેગ અચળાંક અનુક્રમે 1.02×10−4 અને 3.4×10−5S−1 છે તો આ સમયે N2O5 ની સાંદ્રતા કેટલી હશે?
View SolutionRate constant for the reaction 2N_(2)O_(5) to 4NO_(2) + O_(2) is 4.98 xx 10^(-4) s^(-1) . Find the order of reaction?
View SolutionThe rate constant of the reaction, 2N2O5→4NO2+O2 at 300 K is 3×10−5S−1. If the rate of the reaction at the same tamperature is 2.4×10−5moldm−3s−1, then the molar concentration of N2O5 is
View Solution

